Disruption of circadian rhythm as a potential pathogenesis of nocturia
IF 14.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Increasing evidence suggested the multifactorial nature of nocturia, but the true pathogenesis of this condition still remains to be elucidated. Contemporary clinical medications are mostly symptom based, aimed at either reducing nocturnal urine volume or targeting autonomic receptors within the bladder to facilitate urine storage. The day–night switch of the micturition pattern is controlled by circadian clocks located both in the central nervous system and in the peripheral organs. Arousal threshold and secretion of melatonin and vasopressin increase at night-time to achieve high-quality sleep and minimize nocturnal urine production. In response to the increased vasopressin, the kidney reduces the glomerular filtration rate and facilitates the reabsorption of water. Synchronously, in the bladder, circadian oscillation of crucial molecules occurs to reduce afferent sensory input and maintain sufficient bladder capacity during the night sleep period. Thus, nocturia might occur as a result of desynchronization in one or more of these circadian regulatory mechanisms. Disrupted rhythmicity of the central nervous system, kidney and bladder (known as the brain–kidney–bladder circadian axis) contributes to the pathogenesis of nocturia. Novel insights into the chronobiological nature of nocturia will be crucial to promote a revolutionary shift towards effective therapeutics targeting the realignment of the circadian rhythm. The circadian rhythm has a crucial role in regulating nocturia. In this Review, the authors discuss how the brain–kidney–bladder circadian axis can lead to nocturia, prompted by the rationale that understanding these disruptions can lead to developing effective treatments targeting circadian rhythms.
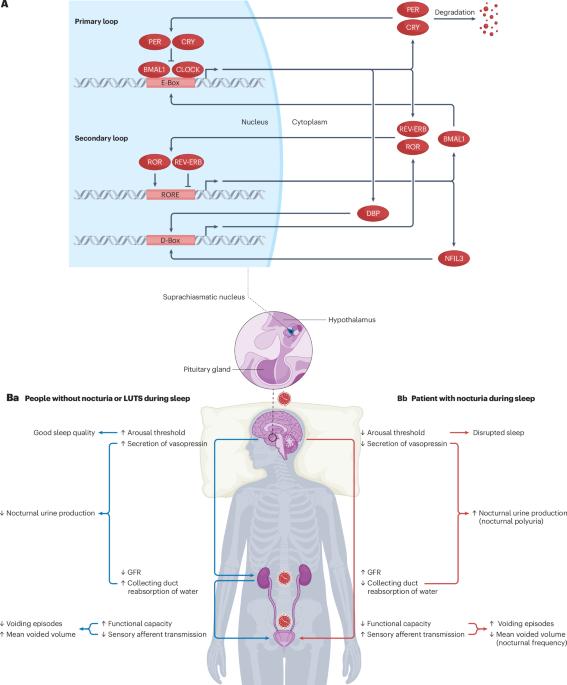
昼夜节律紊乱是夜尿症的潜在发病机制。
越来越多的证据表明,夜尿症具有多因素性质,但其真正的发病机制仍有待阐明。当代临床药物大多以对症治疗为主,旨在减少夜间尿量或针对膀胱内的自主神经受体以促进尿液储存。排尿模式的昼夜转换由位于中枢神经系统和外周器官的昼夜节律钟控制。唤醒阈值以及褪黑激素和血管加压素的分泌在夜间增加,以实现高质量的睡眠并尽量减少夜间尿量。为应对血管加压素的增加,肾脏会降低肾小球滤过率,促进水的重吸收。与此同时,膀胱中的关键分子也会发生昼夜振荡,以减少传入感觉的输入,并在夜间睡眠期间保持足够的膀胱容量。因此,夜尿症的发生可能是其中一种或多种昼夜节律调节机制失调的结果。中枢神经系统、肾脏和膀胱的节律紊乱(称为 "大脑-肾脏-膀胱昼夜轴")是夜尿症的发病机制之一。对夜尿症的时间生物学性质的新认识,对于促进针对昼夜节律调整的有效疗法的革命性转变至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Urology
医学-泌尿学与肾脏学
CiteScore
12.50
自引率
2.60%
发文量
123
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Urology is part of the Nature Reviews portfolio of journals.Nature Reviews' basic, translational and clinical content is written by internationally renowned basic and clinical academics and researchers. This journal targeted readers in the biological and medical sciences, from the postgraduate level upwards, aiming to be accessible to professionals in any biological or medical discipline.
The journal features authoritative In-depth Reviews providing up-to-date information on topics within a field's history and development. Perspectives, News & Views articles, and the Research Highlights section offer topical discussions and opinions, filtering primary research from various medical journals.
Covering a wide range of subjects, including andrology, urologic oncology, and imaging, Nature Reviews provides valuable insights for practitioners, researchers, and academics within urology and related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


