Influence of multiple compression phases during tableting of spray dried Saccharomyces cerevisiae on microbial survival and physical–mechanical tablet properties
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The viability of probiotic microorganisms is essential for their health-promoting effects and must be preserved in the best possible way during the production of the final dosage form, such as tablets. This applies to both drying and tableting. Saccharomyces cerevisiae is spray-dried with suitable protective additives, which were identified in a previous study in which also the influence of the formulation during tableting was investigated. One aspect that has not yet been addressed is the effect of multiple compression, as it is typical with pre- and main compression when using rotary tablet presses. To investigate this, tablets are compressed up to five times. It is shown that when tablet strength and survival are considered together, the application of a pre- and main pressure does not have a significant effect. This facilitates the transferability of findings of compaction studies with a single compression phase. In addition, the data allow to consolidate the mechanism of inactivation of microorganisms during tableting found in previous studies by the same authors. This is based on the porosity reduction, whereby it is shown in the present study that it is irrelevant how this reduction is achieved (change in compression stress or the number of compression cycles).
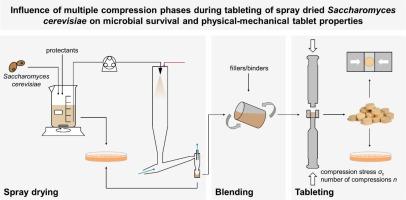
喷雾干燥酵母菌压片过程中的多重压缩阶段对微生物存活率和片剂物理机械性能的影响
益生菌微生物的活力对其促进健康的作用至关重要,因此在生产片剂等最终剂型时,必须以最佳方式保存益生菌微生物的活力。这既适用于干燥,也适用于压片。酵母菌在喷雾干燥过程中使用了合适的保护添加剂,这些添加剂在之前的一项研究中已经确定,该研究还调查了制剂在压片过程中的影响。尚未解决的一个问题是多重压片的影响,因为在使用旋转式压片机时,预压片和主压片是典型的压片方式。为了研究这一点,对片剂进行了多达五次的压片。结果表明,当片剂的强度和存活率被一并考虑时,施加预压和主压的效果并不明显。这有助于将单一压缩阶段的压实研究结果进行推广。此外,这些数据还巩固了同一作者在之前的研究中发现的压片过程中微生物失活的机制。这种机制是基于孔隙率的降低,而本研究表明,孔隙率的降低与实现方式(压缩应力或压缩循环次数的变化)无关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


