Development and biopharmaceutical evaluation of aqueous micelle based corticosteroid formulations for topical treatment of oral lichen planus
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The dearth of approved products to treat oral lichen planus (OLP) compels off-label use of dermatological corticosteroid formulations not optimized for indications in the oral cavity. The aims of this study were to develop aqueous micelle based formulations of triamcinolone acetonide (TA) and fluocinonide (FLU), using D-tocopheryl polyethylene glycol succinate, and to investigate corticosteroid delivery to the epithelium-lamina propria junction region – the anatomical target for OLP treatment – in comparison to that from marketed products. Total mucosal deposition of TA after application of Kenacort® A Orabase® (0.1 % TA) and micellar hydrogel (0.1 %) was 242.1 ± 68.5 and 5936.7 ± 1269.6 ng/cm2, respectively. For FLU, deposition after application of Novoter (0.05 % FLU) and micellar hydrogel (0.05 %) was 617.1 ± 126.5 and 2580.0 ± 285.5 ng/cm2, respectively. A buccal biodistribution study showed that application of micelle hydrogels under occlusion for 30 min delivered 117.0 ± 15.6 ng/cm2 and 225.6 ± 36.7 ng/cm2, of TA and FLU, respectively, to the epithelium-lamina propria junction region. In contrast, the amounts deposited after applying Kenacort® A Orabase® and Novoter, were < LOQ. The results demonstrated that TPGS-based micelles improved mucosal bioavailability of TA and FLU in the epithelium-lamina propria junction region and might serve to improve topical OLP therapy.
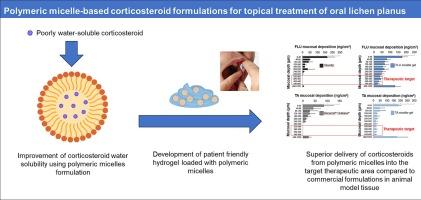
用于局部治疗口腔扁平苔藓的水性胶束皮质类固醇制剂的开发和生物制药评估。
治疗口腔扁平苔藓(OLP)的获批产品匮乏,迫使人们在标签外使用未针对口腔适应症进行优化的皮肤科皮质类固醇制剂。本研究的目的是利用 D-生育酚聚乙二醇琥珀酸酯开发基于水胶束的曲安奈德(TA)和氟西诺奈德(FLU)制剂,并研究皮质类固醇向上皮-固有膜交界区域(OLP 治疗的解剖学目标)的给药情况,并与市场上销售的产品进行比较。使用Kenacort® A Orabase®(0.1% TA)和胶束水凝胶(0.1%)后,TA的总粘膜沉积量分别为242.1 ± 68.5和5936.7 ± 1269.6纳克/平方厘米。对于 FLU,使用 Novoter(0.05 % FLU)和胶束水凝胶(0.05 %)后的沉积量分别为 617.1 ± 126.5 纳克/平方厘米和 2580.0 ± 285.5 纳克/平方厘米。口腔生物分布研究表明,在咬合状态下使用胶束水凝胶 30 分钟后,TA 和 FLU 在上皮-固有膜交界区域的沉积量分别为 117.0 ± 15.6 纳克/平方厘米和 225.6 ± 36.7 纳克/平方厘米。相比之下,使用 Kenacort® A Orabase® 和 Novoter 后的沉积量为
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


