Coamorphous systems of rebamipide: Selection of amino acid coformers based on protein-ligand docking, in vitro assessment and study of interactions by computational and multivariate analysis
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Three coamorphous systems of Rebamipide (REB) with the amino acids namely, Tryptophan (TRP), Phenylalanine (PHE) and Arginine (ARG) are reported. A unique approach for the virtual screening of amino acid coformers is presented by employing molecular docking studies based on interactions of the drug molecule with various amino acid fragments in the drug-receptor cocrystal structure. Successful formation of stable coamorphous systems with ARG, TRP and PHE served as the proof-of-concept along with negative benchmarking standards Histidine and Aspartic acid, wherein coamorphous systems could not be obtained despite multiple trials which resulted in crystalline physical mixtures. The coamorphous systems were characterized by a halo pattern in Powder XRD and a single glass transition temperature (Tg) in modulated DSC. Physical stability assessments of the coamorphous systems showed direct correlation of Tg with the observed stability of the amorphous phase which was found in the order ARGREB > TRPREB ≥ PHEREB. To determine the specific functional groups engaged in the interactions, multivariate analysis was performed on the FTIR spectra. These interactions were further validated by DFT and QTAIM analysis, which revealed key noncovalent interactions in the three coamorphous systems. All three coamorphous systems showed excellent release profiles of the API as demonstrated by the f2 and DE parameters in the order ARGREB ≥ TRPREB > PHEREB ≥ amorphous drug, far exceeding that of the crystalline drug. The interplay of multivariate analysis and QTAIM can be useful in estimating the interactions within the coamorphous systems which can further contribute to stability and physicochemical properties of the systems.
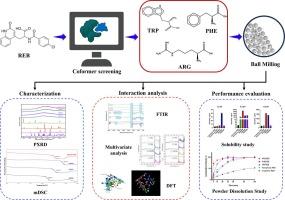
雷巴米特的共形体系:根据蛋白质-配体对接、体外评估以及通过计算和多元分析研究相互作用,选择氨基酸共聚物。
报告了雷巴米特(REB)与色氨酸(TRP)、苯丙氨酸(PHE)和精氨酸(ARG)这三种氨基酸的共晶体系。根据药物分子与药物-受体共晶体结构中各种氨基酸片段的相互作用进行分子对接研究,提出了一种虚拟筛选氨基酸共聚物的独特方法。作为概念验证,ARG、TRP 和 PHE 成功形成了稳定的共晶体系,而负面基准标准组氨酸和天冬氨酸尽管经过多次试验,最终形成了结晶物理混合物,却无法形成共晶体系。共晶体系的特征是粉末 X 射线衍射(Powder XRD)中的光晕模式和调制 DSC 中的单一玻璃化转变温度(Tg)。共晶体系的物理稳定性评估表明,Tg 与观察到的非晶相稳定性直接相关,其顺序为 ARGREB > TRPREB ≥ PHEREB。为了确定参与相互作用的特定官能团,对傅立叶变换红外光谱进行了多元分析。DFT 和 QTAIM 分析进一步验证了这些相互作用,揭示了三种共晶体系中关键的非共价相互作用。从 f2 和 DE 参数来看,这三种共晶体系都显示出良好的原料药释放曲线,其顺序为 ARGREB ≥ TRPREB > PHEREB ≥ 无定形药物,远远超过结晶药物的释放曲线。多变量分析和 QTAIM 的相互作用有助于估计共晶体系内的相互作用,从而进一步提高体系的稳定性和理化性质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


