“Aging” in co-amorphous systems: Dissolution decrease and non-negligible dissolution increase during storage without recrystallization
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Developing co-amorphous systems is a promising strategy to improve the water solubility of poorly water-soluble drugs. Most of the studies focused on the initial dissolution rate of the fresh co-amorphous systems, and only physical stability was investigated after storage. However, the maintenance of the enhanced dissolution rate of co-amorphous systems after storage is necessary for further product development. The maintenance of amorphous forms after storage does not always mean the maintenance of the dissolution rate. In this study, indomethacin, arginine, tryptophan, and phenylalanine were used as the model drug and the co-formers to prepare co-amorphous systems and then stored under dry condition and RH 60 ± 5 % condition. No recrystallization was observed after the storage for 40 d and 80 d. Interestingly, both intrinsic dissolution rate (IDR) decrease and unexpected increase after storage were confirmed. The further mixing of IND and the co-former at a molecular level and the moisture changes of the co-amorphous systems during storage were supposed to play important roles in the aging. This study reminds us that the possible dissolution changes (both dissolution decrease and increase) of co-amorphous systems during storage should be carefully considered, though these samples maintained amorphous forms.
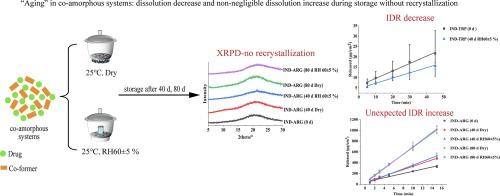
共晶体系的 "老化":在无再结晶的储存过程中,溶解度降低和溶解度增加不可忽略。
开发共晶体系是提高水溶性较差药物水溶性的一种有前途的策略。大多数研究都集中在新鲜共晶体系的初始溶出率上,只调查了储存后的物理稳定性。然而,共晶体系在储存后仍能保持较高的溶出率对于进一步的产品开发是非常必要的。贮藏后非晶态形式的保持并不总是意味着溶出率的保持。本研究使用吲哚美辛、精氨酸、色氨酸和苯丙氨酸作为模型药物和共形物制备共无定形体系,然后在干燥和相对湿度为 60 ± 5 % 的条件下储存。贮存 40 天和 80 天后均未观察到再结晶现象。有趣的是,贮藏后固有溶解速率(IDR)的降低和意外升高都得到了证实。在贮藏过程中,IND 和共聚物在分子水平上的进一步混合以及共晶体系的湿度变化应该在老化过程中发挥了重要作用。这项研究提醒我们,虽然这些样品仍保持非晶态,但应仔细考虑共晶体系在贮藏期间可能发生的溶解变化(溶解度降低和增加)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


