PNSC5325 prevents acute respiratory distress syndrome by alleviating inflammation and inhibiting extracellular matrix degradation of alveolar macrophages
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Background
Acute respiratory distress syndrome (ARDS) is characterized by severe inflammation and significant extracellular matrix (ECM) degradation in the lungs. Our prior research identified the CtBP2-p300-NF-κB (C-terminal-binding protein 2-histone acetyltransferase p300-nuclear factor kappa B) transcriptional complex as critical in ARDS by activating pro-inflammatory cytokine genes.
Methods
An ARDS mouse model was established using intratracheal instillation of lipopolysaccharide (LPS). Small molecules that inhibit the CtBP2-p300 interaction were identified through AlphaScreen. RNA sequencing (RNA-Seq) was conducted to determine differential gene expression. Immunoprecipitation and co-immunoprecipitation analyzed protein interactions. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and immunoblotting detected gene and protein expression. Histological staining evaluated tissue damage.
Results
Through AlphaScreen, two natural compounds, PNSC2477 and PNSC5325, were identified for their ability to inhibit the CtBP2-p300 interaction. While PNSC2477 demonstrated toxicity and was deemed unsuitable for further research, PNSC5325 exhibited minimal toxicity. PNSC5325 effectively inhibited the CtBP2-p300 interaction and reduced pro-inflammatory cytokine gene expression. RNA-Seq analysis of PNSC5325-treated cells indicated significant suppression of pro-inflammatory cytokine genes and matrix metalloproteinases (MMPs). Further molecular studies revealed that the CtBP2-p300 complex, in conjunction with activator protein 1 (AP1), activates MMP expression. PNSC5325 simultaneously suppressed both pro-inflammatory cytokines and MMPs by targeting the CtBP2-p300 complex. In LPS-injected mice, PNSC5325 administration significantly reduced ARDS incidence by inhibiting inflammatory and MMP genes.
Conclusion
These findings suggest that PNSC5325 protects against ARDS by inhibiting key inflammatory and ECM degradation pathways, highlighting its potential as a novel therapeutic agent for ARDS and paving the way for further clinical investigations.
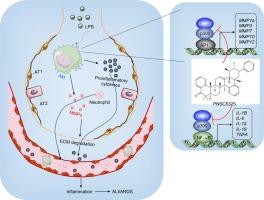
PNSC5325 可通过减轻炎症和抑制肺泡巨噬细胞的细胞外基质降解来预防急性呼吸窘迫综合征。
背景:急性呼吸窘迫综合征(ARDS)以肺部严重炎症和细胞外基质(ECM)显著降解为特征。我们之前的研究发现,CtBP2-p300-NF-κB(C-末端结合蛋白 2-组蛋白乙酰转移酶 p300-核因子卡巴 B)转录复合体通过激活促炎细胞因子基因对 ARDS 起着关键作用:方法:通过气管内灌注脂多糖(LPS)建立了 ARDS 小鼠模型。通过 AlphaScreen 鉴定出抑制 CtBP2-p300 相互作用的小分子。进行 RNA 测序(RNA-Seq)以确定不同的基因表达。免疫沉淀和共免疫沉淀分析了蛋白质的相互作用。逆转录-定量聚合酶链反应(RT-qPCR)和免疫印迹检测基因和蛋白质的表达。组织学染色评估组织损伤:结果:通过 AlphaScreen,发现 PNSC2477 和 PNSC5325 这两种天然化合物具有抑制 CtBP2-p300 相互作用的能力。PNSC2477 具有毒性,不适合进一步研究,而 PNSC5325 的毒性很小。PNSC5325 有效抑制了 CtBP2-p300 的相互作用,并减少了促炎细胞因子基因的表达。对 PNSC5325 处理过的细胞进行的 RNA-Seq 分析表明,促炎细胞因子基因和基质金属蛋白酶(MMPs)受到显著抑制。进一步的分子研究发现,CtBP2-p300 复合物与激活蛋白 1(AP1)共同激活了 MMP 的表达。PNSC5325 通过靶向 CtBP2-p300 复合物,同时抑制了促炎细胞因子和 MMPs。在注射 LPS 的小鼠中,PNSC5325 通过抑制炎症基因和 MMP 基因,显著降低了 ARDS 的发病率:这些研究结果表明,PNSC5325 可通过抑制关键的炎症和 ECM 降解通路来预防 ARDS,突出了其作为 ARDS 新型治疗药物的潜力,并为进一步的临床研究铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


