Effects of immune response on different nano-adjuvants combined with H11 antigen of Haemonchus contortus
IF 4.8
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
The intestinal native protein H11 is one of the most immunodominant antigen of Haemonchus contortus. However, H11 combined with QuilA adjuvant shows only short-lived protection for animals. Nano-adjuvants have the huge potential to extend the immune response in human and animals. Here, we compared the immune responses induced by H11 combined with three nano-adjuvants including lipid nanoparticles (LNP), immunostimulating complex matrix (IMX) and AddaS03™. We measured the cytokine levels of goat PBMCs stimulated by H11 mixed with three nano-adjuvants and QuilA. Results showed that the transcriptions of IL-6, IL-8 and IFN-γ genes were significantly inhibited in all adjuvant group compared with PBS control. H11-IMX combination significantly up-regulated IL-2, IL-17 and TNF-α gene transcriptions. The H11-LNP group showed a significant increase in IL-17 and TNF-α transcriptions, while the H11-AddaS03™ group significantly increased IL-2 transcription. Additionally, mice immunized with these formulations were assessed for splenic lymphocyte proliferation. All H11-adjuvant combinations, except H11-LNP, significantly stimulated lymphocyte proliferation compared to the H11 alone and PBS control groups, with the H11-AddaS03™ combination showing the strongest effect. Analysis of serum antibody levels revealed that all immune groups induced high IgM levels, with H11-IMX inducing the highest IgG levels. Our results demonstrated that IMX or LNP combined with H11 mainly induced a Th17 cell immune response in goat PBMCs, while IMX or AddaS03™ combined with H11 could induce a strong humoral immune response in mice. This study elucidates the immune response profiles of different nano-adjuvant combined with H11 antigen, providing important insights for vaccine development against parasitic nematodes.
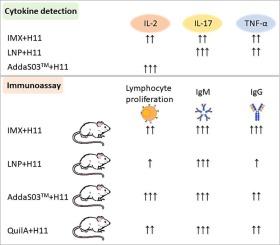
不同纳米佐剂与线虫 H11 抗原结合的免疫反应效应
肠道原生蛋白 H11 是传染性单胞菌(Haemonchus contortus)最具免疫优势的抗原之一。然而,H11 与 QuilA 佐剂结合使用只能对动物产生短暂的保护作用。纳米佐剂在延长人类和动物的免疫反应方面潜力巨大。在这里,我们比较了H11与三种纳米佐剂(包括脂质纳米颗粒(LNP)、免疫刺激复合基质(IMX)和AddaS03™)结合后诱导的免疫反应。我们测定了 H11 与三种纳米佐剂和 QuilA 混合后刺激山羊 PBMC 的细胞因子水平。结果表明,与 PBS 对照组相比,所有佐剂组都能显著抑制 IL-6、IL-8 和 IFN-γ 基因的转录。H11-IMX组合能明显上调IL-2、IL-17和TNF-α基因转录。H11-LNP 组的 IL-17 和 TNF-α 基因转录量明显增加,而 H11-AddaS03™ 组的 IL-2 基因转录量明显增加。此外,还对使用这些制剂免疫的小鼠进行了脾淋巴细胞增殖评估。与单用 H11 组和 PBS 对照组相比,除 H11-LNP 外,所有 H11 佐剂组合都能显著刺激淋巴细胞增殖,其中 H11-AddaS03™ 组合的效果最强。对血清抗体水平的分析表明,所有免疫组都诱导了高水平的 IgM,其中 H11-IMX 诱导的 IgG 水平最高。我们的研究结果表明,IMX 或 LNP 联合 H11 主要诱导山羊 PBMC 的 Th17 细胞免疫反应,而 IMX 或 AddaS03™ 联合 H11 则能诱导小鼠产生强烈的体液免疫反应。本研究阐明了不同纳米佐剂与 H11 抗原结合的免疫应答特征,为寄生线虫疫苗的开发提供了重要启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


