Unveiling the potential of pulmonary surfactant-based nanocarriers for protein inhalation therapy
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-07
DOI:10.1016/j.ejpb.2024.114574
引用次数: 0
Abstract
The study investigates the effect of pulmonary surfactant (PS) coating on the performance of lysozyme-loaded poly(lactic-co-glycolic) acid (PLGA) nanoparticles (NPs). The NPs were fabricated using a double emulsification technique and optimized using the Box-Behnken experimental design (BBED). The NPs were assessed for size, polydispersity index (PDI), zeta potential, drug loading (DL%), and encapsulation efficiency (EE%). In addition, the optimized PLGA NPs were modified with either a neutral dipalmitoylphosphatidylcholine DPPC or an anionic dipalmitoyl phosphatidylglycerol (DPPG) with different molar ratios of PS to PLGA (PS: PLGA = 1:2, 1:1 and 2:1). These NPs were assessed for biological activity, drug release, mucus adhesion, mucus penetration, cellular uptake, toxicity, and in vivo destiny after intratracheal (IT) instillation to mice. Results showed a bi-phasic drug release, with no significant effect of PS on the release and biological activities of PLGA NPs. The PS@PLGA NPs improved mucus adhesion, decreased mucus penetration, and increased cellular internalization of PLGA NPs. In addition, ex vivo experiments demonstrated that DPPC@PLGA NPs and DPPG@PLGA NPs could adhere to mucus. These NPs created a thicker layer at the interface of the airway compared to unmodified PLGA NPs. Moreover, interaction of PS@PLGA NPs with BALF suggested improved mucoadhesive characteristics. Finally, the in vivo studies confirmed the precise distribution of all NPs in the lungs after IT administration. The study presents empirical evidence and scientific guidance for developing a lung surfactant-modified nanocarrier system for lung drug delivery.
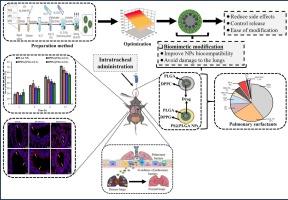
揭示基于肺表面活性物质的纳米载体在蛋白质吸入疗法中的潜力。
本研究探讨了肺表面活性物质(PS)涂层对溶菌酶负载的聚(乳酸-共聚乙醇)酸(PLGA)纳米粒子(NPs)性能的影响。NPs 采用双乳化技术制备,并通过盒式-贝肯实验设计(BBED)进行了优化。对 NPs 的尺寸、多分散指数(PDI)、ZETA 电位、载药量(DL%)和封装效率(EE%)进行了评估。此外,还用中性二棕榈酰基磷脂酰胆碱 DPPC 或阴离子二棕榈酰基磷脂酰甘油 (DPPG) 对优化的 PLGA NPs 进行了修饰,PS 与 PLGA 的摩尔比各不相同(PS: PLGA = 1:2、1:1 和 2:1)。对这些 NPs 进行了生物活性、药物释放、粘液粘附、粘液渗透、细胞摄取、毒性和小鼠气管内(IT)灌注后的体内去向评估。结果表明,PS 对 PLGA NPs 的释放和生物活性无明显影响,药物释放呈双相进行。PS@PLGA NPs 改善了粘液粘附性,降低了粘液穿透性,增加了 PLGA NPs 的细胞内化。此外,体内外实验表明,DPPC@PLGA NPs 和 DPPG@PLGA NPs 可以粘附在粘液上。与未改性的 PLGA NPs 相比,这些 NPs 在气道界面上形成了更厚的一层。此外,PS@PLGA NPs 与 BALF 的相互作用表明它们具有更好的粘液粘附特性。最后,体内研究证实了所有 NPs 在 IT 给药后在肺部的精确分布。该研究为开发用于肺部给药的肺表面活性剂修饰纳米载体系统提供了经验证据和科学指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.
文献相关原料
公司名称
产品信息
上海源叶
Lysozyme
上海源叶
sucrose
上海源叶
DNA
阿拉丁
Rhodamine B

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


