Liposomal Nω-hydroxy-l-norarginine, a proof-of-concept: Arginase inhibitors can be incorporated in liposomes while retaining their therapeutic activity ex vivo
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
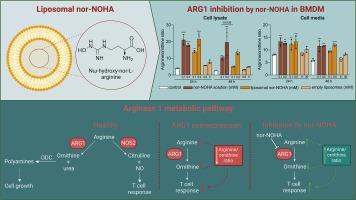
Cancer immunotherapy has evolved significantly over the last decade, with therapeutics targeting the adaptive immune system showing exciting effects in clinics. Yet, the modulation of the innate immune system, particularly the tumor-associated innate immune cells which are an integral part of immune responses in cancer, remains less understood. The arginase 1 (Arg1) pathway is a pivotal metabolic pathway that tumor-associated innate immune cells exploit to create an immunosuppressive tumor microenvironment, leading to the evasion of immune surveillance. The inhibition of Arg1 presents a therapeutic opportunity to reverse this immunosuppression, and Nω‑hydroxy-l-norarginine (nor-NOHA) has emerged as a potent arginase inhibitor with promising in vivo efficacy. However, the rapid systemic clearance of nor-NOHA poses a significant challenge for its therapeutic application. This study pioneers the encapsulation of nor-NOHA in liposomes, aiming to enhance its bioavailability and prolong its inhibitory activity against Arg1. Historically, the extensive interaction between innate immune cells and nanoparticles has been one of the biggest drawbacks in nanomedicine. Here we seek to utilize this effect and deliver liposomal nor-NOHA to the arginase 1 expressing innate immune cells. We systematically investigated the effect of lipid composition, acyl chain length, manufacturing and loading methodology on the encapsulation efficiency (EE%) and release profile of nor-NOHA. Our results indicate that while the manufacturing method and lipid acyl chain length do not significantly impact EE%, they crucially influence the release kinetics of nor-NOHA, with longer acyl chains demonstrating a more sustained release of nor-NOHA from liposomes enabling continuous inhibition of Arg1. Our findings suggest that liposomal nor-NOHA retains its functional inhibitory activity and could offer improved pharmacokinetic properties, making it a compelling base for iterations for further innovative cancer immunotherapeutic strategies in preclinical and clinical evaluations.
脂质体 Nω-羟基-l-去甲精氨酸,概念验证:精氨酸酶抑制剂可纳入脂质体,同时保持其体内外治疗活性。
过去十年来,癌症免疫疗法有了长足的发展,针对适应性免疫系统的疗法在临床上取得了令人振奋的效果。然而,人们对先天性免疫系统,特别是肿瘤相关的先天性免疫细胞的调控仍然知之甚少。精氨酸酶 1(Arg1)通路是肿瘤相关先天性免疫细胞利用的一个关键代谢通路,它能创造免疫抑制性肿瘤微环境,从而逃避免疫监视。抑制 Arg1 为逆转这种免疫抑制提供了治疗机会,Nω-羟基-l-去甲精氨酸(nor-NOHA)已成为一种强效精氨酸酶抑制剂,具有良好的体内疗效。然而,Nor-NOHA 的快速全身清除对其治疗应用构成了重大挑战。本研究开创性地将 nor-NOHA 包裹在脂质体中,旨在提高其生物利用度并延长其对 Arg1 的抑制活性。从历史上看,先天性免疫细胞与纳米粒子之间的广泛相互作用一直是纳米医学的最大缺陷之一。在这里,我们试图利用这一效应,将脂质体 nor-NOHA 递送到表达精氨酸酶 1 的先天性免疫细胞。我们系统地研究了脂质成分、酰基链长度、制造和装载方法对 nor-NOHA 的封装效率(EE%)和释放曲线的影响。我们的结果表明,虽然制造方法和脂质酰基链长度对 EE% 没有显著影响,但它们对 nor-NOHA 的释放动力学有关键影响,酰基链越长,脂质体中 nor-NOHA 的释放越持久,从而能持续抑制 Arg1。我们的研究结果表明,脂质体 nor-NOHA 可保持其功能性抑制活性,并可提供更好的药代动力学特性,使其成为临床前和临床评估中进一步创新癌症免疫治疗策略的令人信服的迭代基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


