Biophysical insight into the interaction mechanism of 4-bromo-N-(thiazol-2-yl)benzenesulfonamide and human serum albumin using multi-spectroscopic and computational studies
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
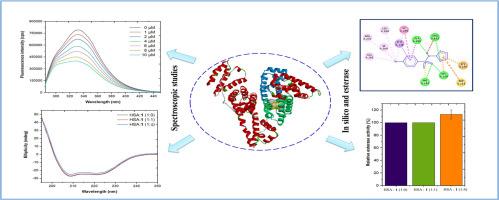
4-Bromo-N-(thiazol-2-yl)benzenesulfonamide (1) is enriched with bioactive components and is highlighted for its pharmacological properties. However, its pharmacokinetic characteristics are yet to be reported. The interaction of compound 1 with carrier proteins in the bloodstream is an important factor that affects its potential therapeutic efficacy. This study aimed to elucidate the pharmacokinetic mechanisms of compound 1 in relation to human serum albumin (HSA) using multi-spectroscopic and computational techniques. Its predicted drug-like properties revealed no mutagenicity, although potential hepatotoxicity and interactions with certain cytochrome P450 enzymes were observed. Spectroscopic analyses extensively provided the interaction between HSA and 1 through a static fluorescence quenching mechanism with spontaneous hydrophobic interactions and hydrogen bonding. The binding constant of the HSA‒1 complex was relatively moderate to strong at a level of 106 M−1. Various spectroscopic techniques including ultraviolet-visible, Fourier transform infrared, and circular dichroism spectroscopies indicated that its binding induced alteration in the α-helix content of HSA. Competitive binding and molecular docking studies designated the preferential binding of 1 to sub-structural domain IIA binding site I of HSA. Molecular dynamic simulations further illustrated the formation of a stable complex between 1 and HSA, accompanied by conformational changes in the protein. Importantly, esterase capacity of the HSA‒1 complex increased compared to the free HSA. Therefore, elucidation of the HSA‒1 binding mechanism provides valuable insights into the pharmacokinetics, suggesting potential benefits for the further development of 1 as a therapeutic agent.
利用多光谱和计算研究对 4-溴-N-(噻唑-2-基)苯磺酰胺与人血清白蛋白相互作用机制的生物物理洞察。
4-溴-N-(噻唑-2-基)苯磺酰胺(1)富含生物活性成分,药理特性突出。然而,其药代动力学特征尚未见报道。化合物 1 与血液中载体蛋白的相互作用是影响其潜在疗效的一个重要因素。本研究旨在利用多光谱和计算技术阐明化合物 1 与人血清白蛋白(HSA)的药动学机制。尽管观察到了潜在的肝毒性和与某些细胞色素 P450 酶的相互作用,但其预测的类药物特性显示没有诱变性。光谱分析广泛提供了 HSA 与 1 之间通过自发疏水相互作用和氢键的静态荧光淬灭机制进行的相互作用。HSA-1 复合物的结合常数在 106 M-1 的水平上属于中等偏强。紫外-可见光谱、傅立叶变换红外光谱和圆二色光谱等多种光谱技术表明,它的结合引起了 HSA α-螺旋含量的改变。竞争性结合和分子对接研究表明,1 优先与 HSA 的亚结构域 IIA 结合位点 I 结合。分子动态模拟进一步表明,1 与 HSA 之间形成了稳定的复合物,并伴随着蛋白质构象的变化。重要的是,与游离的 HSA 相比,HSA-1 复合物的酯酶能力有所提高。因此,阐明 HSA-1 的结合机制为药代动力学提供了有价值的见解,为进一步开发 1 作为治疗药物提供了潜在的益处。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


