Challenges in the development of long acting injectable multivesicular liposomes (DepoFoam® technology)
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-09
DOI:10.1016/j.ejpb.2024.114577
引用次数: 0
Abstract
Multivesicular liposomes (DepoFoam® technology) are distinctive lipid-based sustained release drug delivery systems. Their non-concentric structure differentiates them from unilamellar and multilamellar liposomes. Several products using DepoFoam® technology have been successfully developed and translated into clinical and commercial applications. The unique composition and structure of these particles result in large drug-trap volumes, diverse loading capacities, variable release rates, and different administration routes. With all these advantages, DepoFoam® based products can achieve sustained release pharmacokinetics and significantly improved half-life in various subject species. However, the complexity of constituents and the manufacturing process, as well as the complicated structure and release mechanism, pose challenges to the translation and application of DepoFoam® technology. This review aims to summarize current approved commercial products based on DepoFoam® technology, their structures and components, large-scale manufacturing processes, release characteristics, in vivo pharmacokinetics and clinical outcomes. Challenges in the development and approval of multivesicular liposomes are also highlighted. The persistent academic and industrial research will be needed to overcome the difficulties in developing this unique drug delivery system and pave the path for successful DepoFoam® applications in the future.
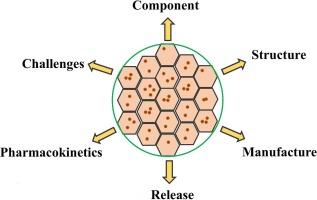
开发长效可注射多囊脂质体(DepoFoam® 技术)的挑战。
多囊脂质体(DepoFoam® 技术)是一种独特的脂基缓释给药系统。它们的非同心结构使其有别于单胶束和多胶束脂质体。采用 DepoFoam® 技术的几种产品已成功开发并投入临床和商业应用。这些微粒的独特组成和结构使其具有较大的药物捕获量、不同的负载能力、可变的释放速率和不同的给药途径。凭借所有这些优势,基于 DepoFoam® 的产品可以实现持续释放药代动力学,并显著改善各种受试物种的半衰期。然而,复杂的成分和生产工艺,以及复杂的结构和释放机理,都给 DepoFoam® 技术的转化和应用带来了挑战。本综述旨在总结目前已批准的基于 DepoFoam® 技术的商业产品、其结构和成分、大规模制造工艺、释放特性、体内药代动力学和临床结果。此外,还重点介绍了多囊脂质体在开发和审批方面面临的挑战。要克服开发这种独特给药系统的困难,并为 DepoFoam® 在未来的成功应用铺平道路,需要坚持不懈的学术和工业研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


