Antifungal peptide-loaded alginate microfiber wound dressing evaluated against Candida albicans in vitro and ex vivo
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-10
DOI:10.1016/j.ejpb.2024.114578
引用次数: 0
Abstract
Invasive fungal infections have high mortality rates, and many current antimycotics are limited by host toxicity and drug resistance. Recent experiments in our laboratory have demonstrated the antifungal activity of dKn2-7, a synthetic peptide, against Candida albicans. The purpose of the current study was to develop a wound dressing capable of dKn2-7 release for extended periods to help combat fungal infection in wounds. dKn2-7 was incorporated into calcium alginate microfibers, an excipient with known wound healing and hemostatic properties. dKn2-7 release rates from the fibers were dependent on drug loading, but all formulations exhibited a burst release with 41–71 % of total theoretical release in the first 15 min and 84–96 % release by 24 h. Calcium release at 15 min was similar to that of a commercial hemostatic dressing, indicating dKn2-7 loading would not adversely affect the hemostatic capability of the alginate fibers. In vitro antifungal studies indicated a dose dependent effect with fibers loaded at ≥20 µg/mg causing significant planktonic killing and ≥30 µg/mg causing significant biofilm killing. Viable fungal counts in biofilms grown on ex vivo porcine skin declined by 99 % following 500 µg/mg fiber treatment. Skin histology indicated no significant differences in tissue damage between treatment groups and controls. Results confirm calcium alginate microfibers are capable of binding and subsequently releasing dKn2-7 over a 24-h period when rehydrated. Furthermore, dKn2-7 released from the fibers was able to significantly reduce biofilms in an ex vivo model with minimal toxicity, indicating these dKn2-7-loaded fiber dressings may be effective at controlling C. albicans biofilm infections in vivo.
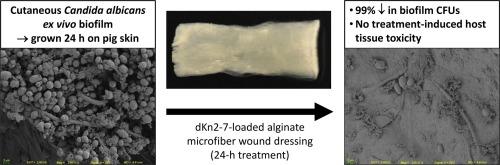
抗真菌肽载体藻酸盐超细纤维伤口敷料对白色念珠菌的体外和体内评估。
侵袭性真菌感染的死亡率很高,而目前许多抗霉菌药物都受到宿主毒性和耐药性的限制。我们实验室最近的实验证明了合成肽 dKn2-7 对白色念珠菌的抗真菌活性。本研究的目的是开发一种能够长时间释放 dKn2-7 的伤口敷料,以帮助对抗伤口中的真菌感染。dKn2-7 被掺入海藻酸钙微纤维中,这是一种具有已知伤口愈合和止血特性的赋形剂。dKn2-7 从纤维中的释放率取决于药物负载量,但所有配方都表现出迸发释放,在最初 15 分钟内释放总量的 41-71%,24 小时内释放 84-96%。15 分钟内的钙释放量与商用止血敷料相似,这表明 dKn2-7 的负载不会对海藻酸纤维的止血能力产生不利影响。体外抗真菌研究表明,≥20 微克/毫克的纤维负载会显著杀死浮游生物,≥30 微克/毫克的纤维负载会显著杀死生物膜,这种效应与剂量有关。经 500 µg/mg 纤维处理后,猪皮肤外生物膜上生长的真菌数量减少了 99%。皮肤组织学显示,处理组与对照组的组织损伤无明显差异。研究结果证实,海藻酸钙微纤维能够与 dKn2-7 结合,并在重新水合后的 24 小时内释放出 dKn2-7。此外,从纤维中释放出的 dKn2-7 能够在体内外模型中显著减少生物膜,且毒性极低,这表明这些装载了 dKn2-7 的纤维敷料可有效控制体内白僵菌生物膜感染。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


