Optimisation of cell fate determination for cultivated muscle differentiation
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
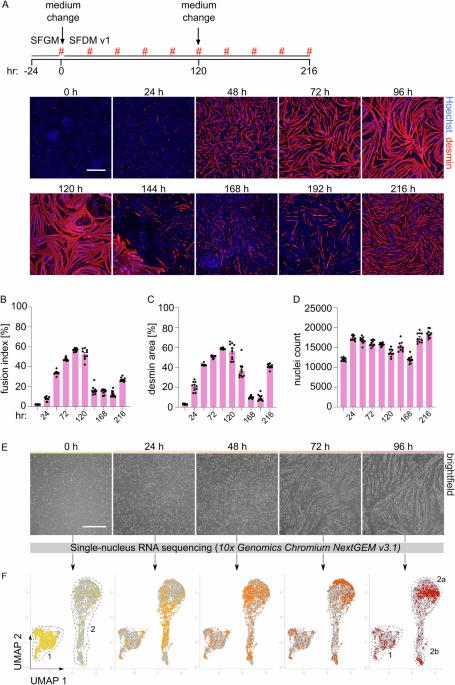
Production of cultivated meat requires defined medium formulations for the robust differentiation of myogenic cells into mature skeletal muscle fibres in vitro. Although these formulations can drive myogenic differentiation levels comparable to serum-starvation-based protocols, the resulting cultures are often heterogeneous, with a significant proportion of cells not participating in myofusion, limiting maturation of the muscle. To address this problem, we employed RNA sequencing to analyse heterogeneity in differentiating bovine satellite cells at single-nucleus resolution, identifying distinct cellular subpopulations including proliferative cells that fail to exit the cell cycle and quiescent ‘reserve cells’ that do not commit to myogenic differentiation. Our findings indicate that the MEK/ERK, NOTCH, and RXR pathways are active during the initial stages of myogenic cell fate determination, and by targeting these pathways, we can promote cell cycle exit while reducing reserve cell formation. This optimised medium formulation consistently yields fusion indices close to 100% in 2D culture. Furthermore, we demonstrate that these conditions enhance myotube formation and actomyosin accumulation in 3D bovine skeletal muscle constructs, providing proof of principle for the generation of highly differentiated cultivated muscle with excellent mimicry to traditional muscle. Single-nucleus RNA sequencing gives insights into heterogeneity during bovine skeletal muscle differentiation, informing the design of improved medium formulations for cultivated meat production.
优化培养肌肉分化的细胞命运决定。
培养肉的生产需要特定的培养基配方,以便在体外将成肌细胞稳健地分化为成熟的骨骼肌纤维。虽然这些培养基配方能使成肌细胞的分化水平与基于血清-饥饿的方案相当,但由此产生的培养物往往是异质性的,有相当一部分细胞不参与肌融合,从而限制了肌肉的成熟。为了解决这个问题,我们利用 RNA 测序技术以单核分辨率分析了分化牛卫星细胞的异质性,确定了不同的细胞亚群,包括未能退出细胞周期的增殖细胞和不参与成肌分化的静止 "储备细胞"。我们的研究结果表明,在决定成肌细胞命运的初始阶段,MEK/ERK、NOTCH 和 RXR 通路非常活跃,通过靶向这些通路,我们可以促进细胞周期的退出,同时减少后备细胞的形成。这种优化的培养基配方能在二维培养中持续产生接近100%的融合指数。此外,我们还证明了这些条件可促进三维牛骨骼肌构建体中肌管的形成和肌动蛋白的积累,为生成与传统肌肉具有极佳相似性的高分化培养肌肉提供了原理证明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


