Multiscale 3D genome rewiring during PTF1A-mediated somatic cell reprogramming into neural stem cells
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
The genome is intricately folded into chromatin compartments, topologically associating domains (TADs) and loops unique to each cell type. How this higher-order genome organization regulates cell fate transition remains elusive. Here we show how a single non-neural progenitor transcription factor, PTF1A, reorchestrates the 3D genome during fibroblast transdifferentiation into neural stem cells (NSCs). Multiomics analyses integrating Hi-C data, PTF1A and CTCF DNA-binding profiles, H3K27ac modification, and gene expression, demonstrate that PTF1A binds to subTAD boundaries subsequently associated with elevated CTCF binding and enhanced boundary insulation, and reorganizes chromatin loops, leading to gene expression changes that drive transdifferentiation into NSCs. Moreover, PTF1A activates enhancers and super-enhancers near low-insulation boundaries and modulates H3K27ac deposition, promoting cell fate transitions. Together, our data implicate an involvement of 3D genome in transcriptional and cell fate alterations, and highlight an essential role for PTF1A in gene expression control and multiscale 3D genome remodeling during cell reprogramming. This study explores the role of the transcription factor PTF1A in remodeling 3D genome architecture during the transdifferentiation of fibroblasts to neural stem cells, highlighting changes in gene expression and TAD organization.
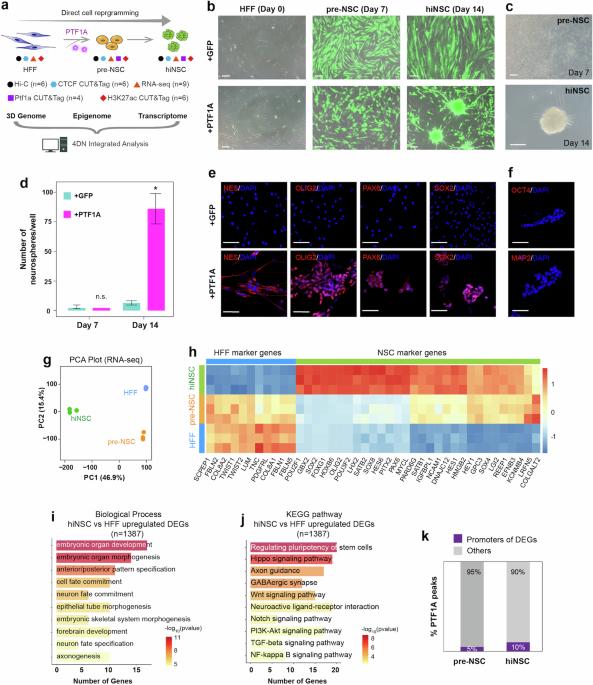
在 PTF1A 介导的体细胞重编程为神经干细胞过程中的多尺度三维基因组重配。
基因组被错综复杂地折叠成染色质区、拓扑关联域(TAD)以及每种细胞类型特有的环路。这种高阶基因组组织如何调控细胞命运的转变仍是一个未知数。在这里,我们展示了单个非神经祖转录因子PTF1A如何在成纤维细胞向神经干细胞(NSCs)转分化过程中重新配置三维基因组。整合了Hi-C数据、PTF1A和CTCF DNA结合图谱、H3K27ac修饰和基因表达的多组学分析表明,PTF1A与subTAD边界结合,随后与CTCF结合增强和边界绝缘增强相关联,并重组染色质环路,导致基因表达变化,推动向NSCs的转分化。此外,PTF1A 还能激活低绝缘边界附近的增强子和超级增强子,并调节 H3K27ac 的沉积,促进细胞命运的转变。总之,我们的数据表明三维基因组参与了转录和细胞命运的改变,并强调了 PTF1A 在细胞重编程过程中基因表达控制和多尺度三维基因组重塑中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


