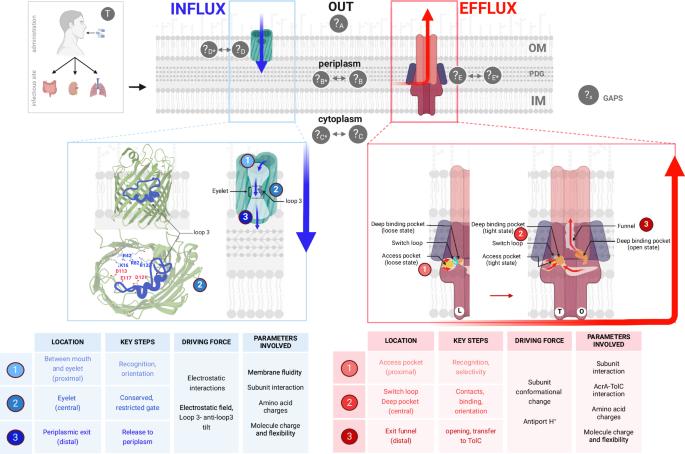
Advances in methods and concepts provide new insight into antibiotic fluxes across the bacterial membrane
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
The sophisticated envelope of Gram-negative bacteria modulates the uptake of small molecules in a side-chain-sensitive manner. Despite intensive theoretical and experimental investigations, a general set of pathways underpinning antibiotic uptake has not been identified. This manuscript discusses the passive influx versus active efflux of antibiotics, considering the responsible membrane proteins and the transported molecules. Recent methods have analyzed drug transport across the bacterial membrane in order to understand their activity. The combination of in vitro, in cellulo and in silico methods shed light on the key, mainly electrostatic, interactions between the molecule surface, porins and transporters during permeation. A key factor is the relationship between the dose of an active compound near its target and its antibacterial activity during the critical early window. Today, methodology breakthroughs provide fruitful tools to precisely dissect drug transport, identify key steps in drug resistance associated with membrane impermeability and efflux, and highlight key parameters to generate more effective drugs. Gram-negative bacteria envelope controls drug accumulation via passive influx and active efflux mechanisms. This article discusses recent in cellulo, in vitro, and in silico methods highlighting key “drug-transporters” dialogues and proposes new perspectives to overcome drug resistance.
方法和概念的进步为了解抗生素在细菌膜上的通量提供了新的视角。
革兰氏阴性细菌复杂的包膜以侧链敏感的方式调节小分子的吸收。尽管进行了深入的理论和实验研究,但仍未找到支撑抗生素吸收的一般途径。本手稿讨论了抗生素的被动流入和主动流出,同时考虑了相关的膜蛋白和转运分子。最近的方法分析了药物在细菌膜上的转运,以了解其活性。体外、细胞内和硅学方法的结合揭示了渗透过程中分子表面、孔蛋白和转运体之间的关键(主要是静电)相互作用。一个关键因素是,在关键的早期窗口期,目标附近的活性化合物剂量与其抗菌活性之间的关系。如今,方法上的突破为精确剖析药物转运、确定与膜不透性和外流相关的耐药性关键步骤以及突出关键参数以产生更有效的药物提供了富有成效的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


