Mechanisms of intestinal DNA damage and inflammation induced by ammonia nitrogen exposure in Litopenaeus vannamei
IF 3.9
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2024-11-08
DOI:10.1016/j.cbpc.2024.110070
引用次数: 0
Abstract
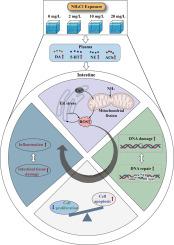
Ammonia nitrogen, a common aquaculture pollutant, harms crustaceans by causing intestinal inflammation, though its exact mechanisms are unclear. Thus, we exposed shrimp to 0, 2, 10 and 20 mg/L NH4Cl exposure for 0, 3, 6, 12, 24, 48, 72 h, and explored the intestinal stress, apoptosis, proliferation, inflammation and its histopathological changes. This research indicated that ammonia nitrogen exposure heightens plasma dopamine (DA), 5-hydroxytryptamine (5-HT), norepinephrine (NE), and acetylcholine (ACh) levels, alters gene expression of neurotransmitter receptors in the intestine, triggering the PLC![]() Ca2+ pathway and induces endoplasmic reticulum stress. Additionally, mitochondrial fission-related genes (Drp1, FIS1) significantly increase, the level of reactive oxygen species (ROS) was significantly elevated in the intestine, which induced DNA damage effects and initiated the DNA repair function, mainly through the base excision repair pathway, but with a low repair efficiency. By determining the expression of key genes of caspase-dependent and non-caspase-dependent apoptotic pathways, it was found that ammonia nitrogen exposure induced apoptosis in intestinal cells, proliferation key signaling pathways such as Wnt, EGFR and FOXO signaling showed an overall decrease after ammonia nitrogen exposure, combined with the gene expression of cell cycle proteins and proliferation markers, indicated that the proliferation of intestinal cells was inhibited. Performing pearson correlation analysis of intestinal cell damage, proliferation, and inflammatory factors, we hypothesized that ammonia nitrogen exposure induces intestinal endoplasmic reticulum stress and mitochondrial fission, induces elevated ROS, leads to DNA damage, and causes inflammation and damage in intestinal tissues by the underlying mechanism of promoting apoptosis and inhibiting proliferation.
Ca2+ pathway and induces endoplasmic reticulum stress. Additionally, mitochondrial fission-related genes (Drp1, FIS1) significantly increase, the level of reactive oxygen species (ROS) was significantly elevated in the intestine, which induced DNA damage effects and initiated the DNA repair function, mainly through the base excision repair pathway, but with a low repair efficiency. By determining the expression of key genes of caspase-dependent and non-caspase-dependent apoptotic pathways, it was found that ammonia nitrogen exposure induced apoptosis in intestinal cells, proliferation key signaling pathways such as Wnt, EGFR and FOXO signaling showed an overall decrease after ammonia nitrogen exposure, combined with the gene expression of cell cycle proteins and proliferation markers, indicated that the proliferation of intestinal cells was inhibited. Performing pearson correlation analysis of intestinal cell damage, proliferation, and inflammatory factors, we hypothesized that ammonia nitrogen exposure induces intestinal endoplasmic reticulum stress and mitochondrial fission, induces elevated ROS, leads to DNA damage, and causes inflammation and damage in intestinal tissues by the underlying mechanism of promoting apoptosis and inhibiting proliferation.
氨氮暴露诱发万年青肠道 DNA 损伤和炎症的机制
氨氮是一种常见的水产养殖污染物,通过引起肠道炎症对甲壳类动物造成危害,但其确切机制尚不清楚。因此,我们将对虾暴露于 0、2、10 和 20 mg/L NH4Cl 下 0、3、6、12、24、48、72 h,并探究其肠道应激、凋亡、增殖、炎症及其组织病理学变化。研究表明,氨氮暴露可提高血浆中多巴胺(DA)、5-羟色胺(5-HT)、去甲肾上腺素(NE)和乙酰胆碱(ACh)的水平,改变肠道中神经递质受体的基因表达,触发 PLCCa2+ 通路,诱导内质网应激。此外,线粒体裂变相关基因(Drp1、FIS1)明显增加,肠道内活性氧(ROS)水平显著升高,诱发DNA损伤效应,启动DNA修复功能,主要通过碱基切除修复途径,但修复效率较低。通过测定caspase依赖性和非caspase依赖性凋亡通路关键基因的表达,发现氨氮暴露诱导肠道细胞凋亡,增殖关键信号通路如Wnt、EGFR和FOXO信号通路在氨氮暴露后整体下降,结合细胞周期蛋白和增殖标志物的基因表达,表明肠道细胞的增殖受到抑制。通过对肠道细胞损伤、增殖和炎症因子进行皮尔逊相关分析,我们推测氨氮暴露会诱导肠道内质网应激和线粒体裂解,诱导ROS升高,导致DNA损伤,并通过促进凋亡和抑制增殖的内在机制引起肠道组织的炎症和损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


