Preparation of N-(2-hydroxypropyltrimonium chloride)-O-dodecylylpyridine chitosan quaternary ammonium salt and its antibacterial activities
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Chitosan derivatives, including O-carboxymethyl chitosan (CMC), N-(2-hydroxypropyltrimonium chloride)-O-carboxymethyl chitosan (QCMC), and N-(2-hydroxypropyltrimonium chloride)-O-dodecylylpyridine chitosan quaternary ammonium salt (DQCMC), were synthesized and characterized using Fourier transform infrared (FTIR) spectroscopy, 1H nuclear magnetic resonance (1H NMR) spectroscopy, Ultraviolet–visible (UV–vis) spectroscopy, and element analysis (EA). The antibacterial activities of chitosan and chitosan derivatives against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were evaluated through the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and antibacterial rate assays. Results demonstrated that DQCMC exhibited significantly higher antibacterial efficacy compared to chitosan, CMC, and QCMC. The MIC of DQCMC against E. coli and S. aureus were 31 μg/mL and 7 μg/mL, respectively, with a 100 % antibacterial rate at a concentration of 0.5 mg/mL. Furthermore, assessment of mouse fibroblast (L929) cell viability using cell counting kit-8 (CCK-8) methods revealed no toxicity associated with the material.
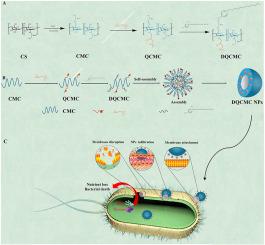
N-(2-羟基丙基三甲基氯化铵)-O-十二烷基吡啶壳聚糖季铵盐的制备及其抗菌活性。
合成了壳聚糖衍生物,包括 O-羧甲基壳聚糖(CMC)、N-(2-羟基丙基三甲基氯化铵)-O-羧甲基壳聚糖(QCMC)和 N-(2-羟基丙基三甲基氯化铵)-O-十二烷基吡啶壳聚糖季铵盐(DQCMC)、合成了壳聚糖季铵盐(DQCMC),并使用傅立叶变换红外光谱(FTIR)、1H 核磁共振(1H NMR)光谱、紫外可见光谱(UV-vis)和元素分析(EA)对其进行了表征。通过最低抑菌浓度(MIC)、最低杀菌浓度(MBC)和抗菌率测定,评估了壳聚糖和壳聚糖衍生物对大肠杆菌(E. coli)和金黄色葡萄球菌(S. aureus)的抗菌活性。结果表明,与壳聚糖、CMC 和 QCMC 相比,DQCMC 的抗菌效力明显更高。DQCMC 对大肠杆菌和金黄色葡萄球菌的 MIC 分别为 31 μg/mL 和 7 μg/mL,浓度为 0.5 mg/mL 时的抗菌率为 100%。此外,使用细胞计数试剂盒-8(CCK-8)方法对小鼠成纤维细胞(L929)的存活率进行评估后发现,该材料没有毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


