Effects of NMDA glutamatergic receptors pharmacological stimulation of the ventral tegmental area on the memory deficits induced by maternal deprivation
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Maternal deprivation (MD) is a potent stressor during early life and can lead to behavioral changes during adulthood. Several neurochemical mechanisms underlying MD-induced stress have been proposed; among them is the damage caused to dopaminergic neurons in the ventral tegmental area (VTA). We hypothesized that pharmacological stimulation of dopaminergic neurons in VTA by the infusion of an N-Methyl-D-Aspartate (NMDA) receptors agonist (used considering the wide distribution of these glutamatergic receptors in the VTA neurons) can reverse MD-induced memory deficits. Here, we demonstrated that MD affects male and female rats distinctly, with females being more resilient to early-life stress. Furthermore, NMDA pharmacological stimulation of the VTA promotes object recognition (OR) memory persistence in male and female non-MD rats. In males, infusion of NMDA into the VTA immediately after the learning session reverses recognition memory deficits related to MD. Although MD female rats have not shown deficits in OR memory consolidation, the NMDA infusion immediately after the learning session promotes memory persistence. We verified that MD leads to memory deficits in adult male rats, while the females are resilient to early life stress. Furthermore, NMDA pharmacological stimulation of dopaminergic VTA neurons reveals the dopaminergic modulation of OR memory in MD rats, even in females that did not exhibit memory deficits.
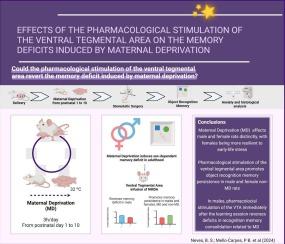
对腹侧被盖区 NMDA 谷氨酸能受体的药理刺激对母体剥夺引起的记忆缺陷的影响。
母性剥夺(MD)是生命早期的一种强效应激源,可导致成年期的行为变化。目前已提出了几种MD诱导应激的神经化学机制,其中包括对腹侧被盖区(VTA)多巴胺能神经元的损伤。我们假设,通过输注 N-甲基-D-天冬氨酸(NMDA)受体激动剂(考虑到这些谷氨酸能受体广泛分布于 VTA 神经元中)对 VTA 中的多巴胺能神经元进行药理刺激,可以逆转 MD 诱导的记忆缺陷。在这里,我们证明了 MD 对雄性和雌性大鼠的影响是不同的,雌性大鼠对早期生活压力的适应能力更强。此外,对VTA的NMDA药理刺激可促进雄性和雌性非MD大鼠的物体识别(OR)记忆持久性。在雄性大鼠中,学习课程结束后立即向VTA注入NMDA可逆转与MD相关的识别记忆缺陷。虽然MD雌性大鼠在OR记忆巩固方面没有表现出缺陷,但在学习后立即注入NMDA可促进记忆的持久性。我们证实,MD 会导致成年雄性大鼠出现记忆缺陷,而雌性大鼠对早期生活压力具有恢复能力。此外,对多巴胺能VTA神经元的NMDA药理刺激揭示了多巴胺能对MD大鼠OR记忆的调节作用,即使是没有表现出记忆缺陷的雌性大鼠也是如此。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


