HMGB2 promotes smooth muscle cell proliferation through PPAR-γ/PGC-1α pathway-mediated glucose changes in aortic dissection
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background and aims
Aortic dissection (AD) is a fatal condition with a complicated pathogenesis. High mobility group protein B2 (HMGB2) is a member of the high mobility group protein family; HMGB2 is involved in innate immunity and inflammatory diseases, but its role in AD remains unclear.
Methods
HMGB2−/− mice were generated and treated with β-aminopropionitrile and angiotensin II (Ang II) to establish an AD model. An F12 gel containing AAV9-HMGB2 was applied to overexpress HMGB2 in mice. Pathological changes in the aorta were assessed by visualizing vascular collagen deposition and elastic fiber fracture via H&E, Masson and EVG staining. HMGB2 expression was measured by Western blotting and immunohistochemistry. MTS, CCK-8 and EdU assays were used to test cell proliferation.
Results
HMGB2 expression was increased in samples from AD patients, samples from AD mouse modeland human aortic smooth muscle cells (HASMCs). HMGB2 promoted HASMC proliferation. Immunofluorescence staining and plasma membrane protein isolation revealed that HMGB2 decreased GLUT1 expression and promoted GLUT4 translocation. HMGB2 was also found to inhibit the expression of SIRT1/PGC-1α, but blocking the PPAR-γ pathway attenuated this effect. HMGB2−/− significantly reduced the incidence and mortality rates of AD, whereas treatment with AAV9-HMGB2 exacerbated AD.
Conclusions
This study suggests that HMGB2 promotes HASMC proliferation and vascular remodeling by regulating glucose metabolism through the PPAR-γ/SIRT1/PGC-1α pathway. HMGB2 knockdown reduces, while HMGB2 overexpression promotes, the occurrence of AD in mice. This study may help elucidate the underlying mechanisms and provide a new preventive target for AD.
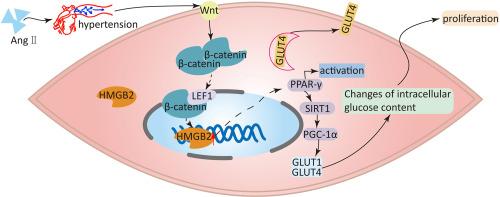
在主动脉夹层中,HMGB2 通过 PPAR-γ/PGC-1α 通路介导的葡萄糖变化促进平滑肌细胞增殖。
背景和目的:主动脉夹层(AD)是一种致命疾病,发病机制复杂。高迁移率基团蛋白 B2(HMGB2)是高迁移率基团蛋白家族的成员;HMGB2 参与先天性免疫和炎症性疾病,但其在 AD 中的作用仍不清楚。方法:产生 HMGB2-/-小鼠,并用 β-氨基丙腈和血管紧张素 II(Ang II)处理,以建立 AD 模型。应用含有 AAV9-HMGB2 的 F12 凝胶在小鼠体内过表达 HMGB2。通过H&E、Masson和EVG染色观察血管胶原沉积和弹性纤维断裂,评估主动脉的病理变化。通过 Western 印迹和免疫组化检测 HMGB2 的表达。采用 MTS、CCK-8 和 EdU 检测法检测细胞增殖:结果:AD 患者样本、AD 小鼠模型样本和人主动脉平滑肌细胞(HASMCs)中 HMGB2 的表达均有所增加。HMGB2 促进了 HASMC 的增殖。免疫荧光染色和质膜蛋白分离显示,HMGB2 降低了 GLUT1 的表达并促进了 GLUT4 的转位。研究还发现,HMGB2 可抑制 SIRT1/PGC-1α 的表达,但阻断 PPAR-γ 通路可减轻这种影响。HMGB2-/-能显著降低AD的发病率和死亡率,而用AAV9-HMGB2治疗则会加重AD:本研究表明,HMGB2 通过 PPAR-γ/SIRT1/PGC-1α 途径调节葡萄糖代谢,从而促进 HASMC 增殖和血管重塑。敲除 HMGB2 可减少小鼠 AD 的发生,而过表达 HMGB2 则会促进 AD 的发生。这项研究可能有助于阐明其潜在机制,并为AD提供一个新的预防靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis
医学-外周血管病
CiteScore
9.80
自引率
3.80%
发文量
1269
审稿时长
36 days
期刊介绍:
Atherosclerosis has an open access mirror journal Atherosclerosis: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atherosclerosis brings together, from all sources, papers concerned with investigation on atherosclerosis, its risk factors and clinical manifestations. Atherosclerosis covers basic and translational, clinical and population research approaches to arterial and vascular biology and disease, as well as their risk factors including: disturbances of lipid and lipoprotein metabolism, diabetes and hypertension, thrombosis, and inflammation. The Editors are interested in original or review papers dealing with the pathogenesis, environmental, genetic and epigenetic basis, diagnosis or treatment of atherosclerosis and related diseases as well as their risk factors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


