Subcellular mass spectrometric detection unveils hyperglycemic memory in the diabetic heart
Abstract
Background
Intensive glycemic control is insufficient to reduce the risk of heart failure in patients with diabetes mellitus. While the hyperglycemic memory in the diabetic cardiomyopathy has been well documented, its underlying mechanisms are not fully understood. The present study tried to investigate whether the dysregulated proteins/biological pathways, which persistently altered in diabetic hearts during normoglycemia, participate in the hyperglycemic memory.
Methods
Hearts of streptozotocin-induced diabetic mice, with or without intensive glycemic control using slow-release insulin implants, were collected. Proteins from total heart samples and subcellular fractions were assessed by mass spectrometry, Western blotting, and KEGG pathway enrichment analysis. mRNA sequencing was used to determine whether the persistently altered proteins were regulated at the transcriptional or post-transcriptional level.
Results
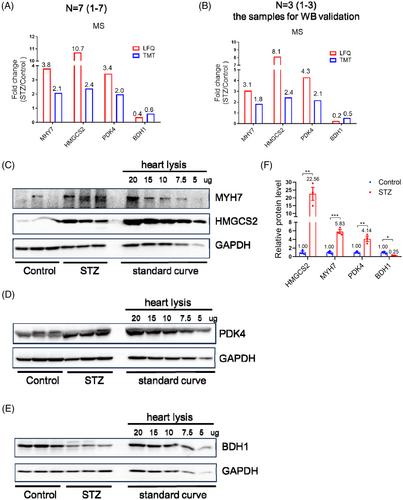
Western blot validation of several proteins with high pathophysiological importance, including MYH7, HMGCS2, PDK4, and BDH1, indicated that mass spectrometry was able to qualitatively, but not quantitatively, reflect the fold changes of certain proteins in diabetes. Pathway analysis revealed that the peroxisome, PPAR pathway, and fatty acid metabolism could be efficiently rescued by glycemic control. However, dysregulation of oxidative phosphorylation and reactive oxygen species persisted even after normalization of hyperglycemia. Notably, mRNA sequencing revealed that dysregulated proteins in the oxidative phosphorylation pathway were not accompanied by coordinated changes in mRNA levels, indicating post-transcriptional regulation. Moreover, literature review and bioinformatics analysis suggested that hyperglycemia-induced persistent alterations of miRNAs targeted genes from the persistently dysregulated oxidative phosphorylation pathway, whereas, oxidative phosphorylation dysfunction-induced ROS regulated miRNA expression, which thereby might sustained the dysregulation of miRNAs.
Conclusions
Glycemic control cannot rescue hyperglycemia-induced alterations of subcellular proteins in the diabetic heart, and persistently altered proteins are involved in multiple functional pathways, including oxidative phosphorylation. These findings might provide novel insights into hyperglycemic memory in diabetic cardiomyopathy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


