Directed nucleophilic aromatic substitution reaction†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In this study, we discovered a directed nucleophilic aromatic substitution reaction, “directed SNAr (dSNAr),” in the reaction of ortho-iodobezamides and amine in the presence of pyridine. The reaction proceeded ortho-specifically and did not require a strong electron-withdrawing group on the arene substrate. Most reactions proceeded at room temperature in the presence of Py, and a wide range of amine nucleophiles can be applied. Furthermore, the reactions with benzamide substituted with multiple halogens were found to be 100% ortho-selective.
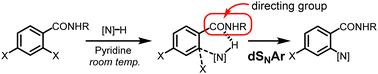
定向亲核芳香取代反应。
在这项研究中,我们发现了一种定向亲核芳香取代反应,即 "定向 SNAr (dSNAr)",它是在吡啶存在下,正交碘代氮杂酰胺与胺的反应。该反应以正交特异性方式进行,不需要炔基质上的强夺电子基团。大多数反应都是在室温、有 Py 存在的情况下进行的,而且可以使用多种胺类亲核物。此外,还发现与多个卤素取代的苯甲酰胺的反应具有 100% 的正选择性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


