Predictive Prognostic Model for Hepatocellular Carcinoma Based on Seven Genes Participating in Arachidonic Acid Metabolism
Abstract
Background
The occult onset and rapid progression of hepatocellular carcinoma (HCC) lead to an unsatisfactory overall survival (OS) rate. Established prognostic predictive models based on tumor-node-metastasis staging and predictive factors do not report satisfactory predictive efficacy. Arachidonic acid plays pivotal roles in biological processes including inflammation, regeneration, immune modulation, and tumorigenesis. We, therefore, constructed a prognostic predictive model based on seven genes linked to arachidonic acid metabolism, using samples of HCC patients from databases to analyze the genomic profiles. We also assessed the predictive stability of the constructed model.
Methods
Sample data of 365 patients diagnosed with HCC were extracted from The Cancer Genome Atlas (TCGA, training set) and HCCDB18, GSE14520, and GSE76427 databases (validation sets). Patient samples were clustered using ConsensusClusterPlus analysis based on the expression levels of 12 genes involved in arachidonic acid metabolism that were significantly associated with HCC prognosis. Differentially expressed genes (DEGs) within different clusters were distinguished and compared using WebGestaltR. Immunohistochemistry (IHC) analysis was performed using a human HCC tissue microarray (TMA). Tumor immune microenvironment assessment was performed using ESTIMATE, ssGSEA, and TIDE.
Results
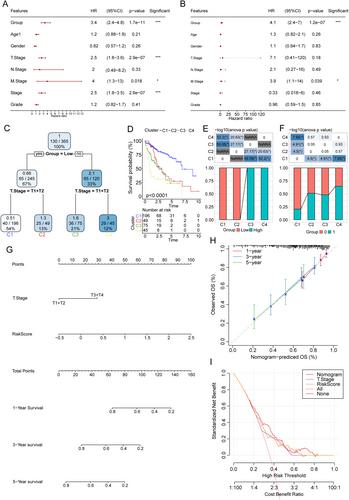
Samples of patients with HCC were classified into three clusters, with significant differences in OS. Cluster 2 showed the best prognosis, whereas cluster 1 presented the worst. The three clusters showed significant differences in immune infiltration. We then performed Cox and LASSO regression analyses, which revealed CYP2C9, G6PD, CDC20, SPP1, PON1, TRNP1, and ADH4 as prognosis-related hub genes, making it a simplified prognostic model. TMA analysis for the seven target genes showed similar results of regression analyses. The high-risk group showed a significantly worse prognosis and reduced immunotherapy efficacy. Our model showed stable prognostic predictive efficacy.
Conclusions
This seven-gene–based model showed stable outcomes in predicting HCC prognosis as well as responses to immunotherapy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


