Phase 2 Trial of Enfortumab Vedotin in Patients With Previously Treated Locally Advanced or Metastatic Urothelial Carcinoma in China
Abstract
Background
Enfortumab vedotin, a fully human monoclonal antibody–drug conjugate (ADC) directed to Nectin-4, prolonged overall survival (OS) versus standard chemotherapy in patients with previously treated locally advanced or metastatic urothelial carcinoma (mUC) previously receiving a programmed cell death protein 1/ligand 1 (PD-1/L1) inhibitor and platinum-based chemotherapy in the pivotal, phase 3 EV-301 clinical trial, supporting global approvals of enfortumab vedotin monotherapy. This bridging study was the first to evaluate enfortumab vedotin monotherapy in previously treated Chinese patients with locally advanced or mUC.
Methods
EV-203 was a multicenter, open-label, phase 2 study (NCT04995419) assessing efficacy, safety/tolerability, pharmacokinetics (PK), and immunogenicity of enfortumab vedotin in 40 Chinese patients (PK analysis set, n = 13) with previously treated locally advanced or mUC. Patients received enfortumab vedotin 1.25 mg/kg (Days 1, 8, and 15). Primary endpoints included confirmed objective response rate (ORR) by the independent review committee (IRC) and PK parameters of ADC, total antibody (TAb), and free monomethyl auristatin E (MMAE). Secondary endpoints included investigator-assessed confirmed ORR; investigator-/IRC-assessed duration of response (DOR), disease control rate (DCR), and progression-free survival (PFS); OS; immunogenicity; and safety/tolerability.
Results
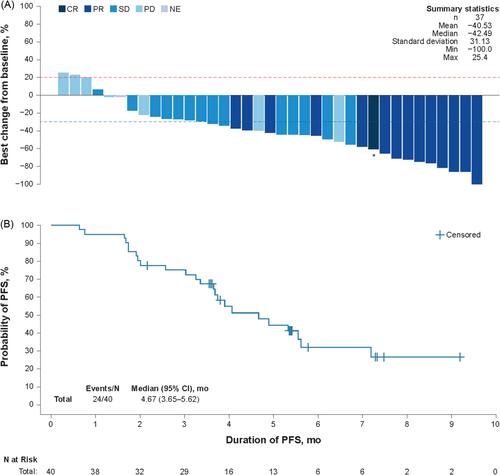
As of May 13, 2022, the median follow-up was 6.5 months. Confirmed ORR was 37.5% (n/N = 15/40; 95% CI: 22.7%–54.2%) by IRC and 42.5% (n/N = 17/40; 95% CI: 27.0%–59.1%) by investigator assessment. By IRC, DCR was 72.5% (n = 29), median DOR was not reached, and median PFS was 4.7 months. Median OS was not reached. Endpoints assessed by investigators were consistent with IRC assessments. Two patients discontinued treatment for treatment-related adverse events. No new safety signals were identified. ADC, TAb, and free MMAE were characterized in Chinese patients and consistent with previously characterized populations. The incidence of positive antitherapeutic antibodies postbaseline was 0%.
Conclusion
Enfortumab vedotin demonstrated meaningful clinical activity with a manageable safety profile in Chinese patients with previously treated locally advanced or mUC.
Trial Registration
ClinicalTrials.gov identifier: NCT04995419
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


