Optimization of a high throughput screening platform to identify inhibitors of asymmetric diadenosine polyphosphatases
IF 2.6
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
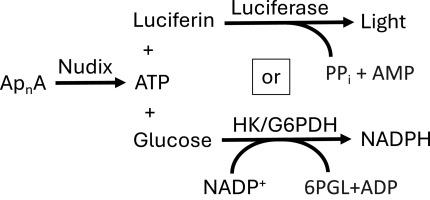
When stressed, cells synthesize di-adenosine polyphosphates (ApnA), and cellular organisms also express proteins that degrade these compounds to release ATP. Most of these proteins are members of the nudix hydrolase superfamily, and several are involved in bacterial pathogenesis, neurodevelopment, and cancer. The goal of this project is to assist in the discovery of inhibitors of these enzymes that could be used to study ApnA function and the cellular role of these nudix enzymes. Because these enzymes cleave Ap4A and Ap5A to produce ATP, two standard ATP detection techniques were optimized and compared here for their suitability for high throughput screening. In the first assay, cleavage is monitored by coupling to a reaction catalyzed by firefly luciferase. In the second assay, cleavage is detected by coupling to hexokinase, glucose 6-phosphate dehydrogenase, and diaphorase. Although the former assay was more sensitive, the latter was more reproducible, linear, and suitable for screening and kinetic analyses. The assays were used to characterize the kinetics of reactions catalyzed by various nudix enzymes isolated from E. coli, humans, and Mycobacterium tuberculosis, the bacterium that causes tuberculosis. Results reveal subtle differences between the proteins that might be exploited to identify specific small molecule inhibitors.
优化高通量筛选平台,以确定不对称二腺苷多磷酸酶的抑制剂。
当受到压力时,细胞会合成二腺苷多磷酸盐(ApnA),细胞生物体也会表达降解这些化合物以释放 ATP 的蛋白质。这些蛋白质大多属于 nudix 水解酶超家族,其中有几种涉及细菌致病、神经发育和癌症。本项目的目标是协助发现这些酶的抑制剂,用于研究 ApnA 的功能和这些 nudix 酶的细胞作用。由于这些酶会裂解 Ap4A 和 Ap5A 以产生 ATP,因此对两种标准 ATP 检测技术进行了优化和比较,以确定它们是否适用于高通量筛选。在第一种检测方法中,通过与萤火虫荧光素酶催化的反应耦合来监测裂解。在第二种检测方法中,通过与己糖激酶、6-磷酸葡萄糖脱氢酶和二磷酸盐酶偶联来检测裂解。虽然前一种检测方法更灵敏,但后一种检测方法的可重复性和线性更好,适合筛选和动力学分析。这些检测方法被用来描述从大肠杆菌、人类和结核分枝杆菌(导致结核病的细菌)中分离出来的各种 nudix 酶催化反应的动力学特征。研究结果揭示了蛋白质之间的细微差别,可以利用这些差异找出特定的小分子抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


