Preparation of calcium-based phosphate adsorbent and mineral-rich humic acid fertilizer from biomass ash and bamboo by hydrothermal-pyrolysis: Performance and mechanism
IF 7.7
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Biomass ash (BA) contains alkaline cations such as K, Ca, and Mg. Due to its high pH, direct application to the soil may result in soil salinization. Composting of BA with organic matter is an effective strategy, but the composting cycle is long and there is a large amount of insoluble residue in the product. Therefore, this research proposed for the first time using the hydrothermal method to rapidly convert BA and bamboo powder (BP) into water - soluble fertilizer (WSF) within 4 h. The insoluble hydrothermal residue was further converted into calcium - rich biochar phosphorus adsorption material by a simple pyrolysis process. WSF was neutral and contained humic acid and elements like K, Ca, Mg, and Si. A 14 - day wheat hydroponic experiment showed that the addition of 0.0125% WSF increased the fresh weight of wheat by 18.77% compared with deionized water. The calcium - based biochar adsorbent produced by pyrolysis had an ideal adsorption capacity of up to 113.6 mg P g−1 for phosphate in water, higher than many existing reports. The adsorption mechanisms mainly included surface precipitation, ion exchange, and electrostatic attraction. Moreover, the calcium - rich biochar sample slowly released phosphorus into water after adsorbing phosphate. When the pH was 3 or 4, the removal rate of Pb2+, Cd2+, and Cu2+ at 15 – 20 mg L−1 was as high as 99%. This indicated its potential as a slow - release fertilizer and heavy metal remediation agent. This research provided a new way of thinking for the treatment and disposal of BA.
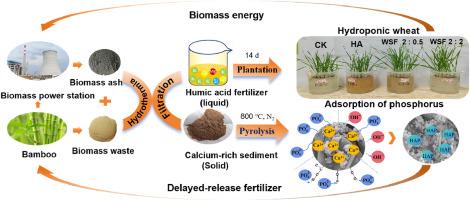
以生物质灰烬和竹子为原料,通过水热热解法制备钙基磷酸盐吸附剂和富含矿物质的腐植酸肥料:性能与机理。
生物质灰(BA)含有钾、钙和镁等碱性阳离子。由于其 pH 值较高,直接施用到土壤中可能会导致土壤盐碱化。将生物质灰与有机物堆肥是一种有效的策略,但堆肥周期长,产品中存在大量不溶性残留物。因此,本研究首次提出利用水热法在 2 小时内将 BA 和竹粉(BP)快速转化为水溶性肥料(WSF)。通过简单的热解过程,不溶性水热残渣进一步转化为富含钙的生物炭磷吸附材料。WSF 呈中性,含有腐殖酸和钾、钙、镁、硅等元素。一项为期 14 天的小麦水培实验表明,与去离子水相比,添加 0.0125% 的 WSF 可使小麦鲜重增加 18.77%。热解产生的钙基生物炭吸附剂对水中磷酸盐的理想吸附容量高达 113.6mg P g-1,高于许多现有报告。吸附机理主要包括表面沉淀、离子交换和静电吸引。此外,富钙生物炭样品在吸附磷酸盐后会缓慢地将磷释放到水中。当 pH 值为 3 或 4 时,Pb2+、Cd2+ 和 Cu2+ 在 15 - 20 mg L-1 的去除率高达 99%。这表明它具有作为缓释肥料和重金属修复剂的潜力。这项研究为生物碱的处理和处置提供了一种新思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Research
环境科学-公共卫生、环境卫生与职业卫生
CiteScore
12.60
自引率
8.40%
发文量
2480
审稿时长
4.7 months
期刊介绍:
The Environmental Research journal presents a broad range of interdisciplinary research, focused on addressing worldwide environmental concerns and featuring innovative findings. Our publication strives to explore relevant anthropogenic issues across various environmental sectors, showcasing practical applications in real-life settings.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


