Non-invasive ultra-sensitive detection of breast cancer biomarker using cerium nanoparticle functionalized graphene oxide enabled impedimetric aptasensor
IF 10.5
1区 生物学
Q1 BIOPHYSICS
引用次数: 0
Abstract
Epidermal growth factor receptor (EGFR) is a transmembrane protein and a key biomarker implicated in the pathogenesis of breast cancer. Early and precise detection of EGFR is crucial for effective diagnosis, prognosis, and therapeutic intervention. However, conventional EGFR detection techniques, such as biopsy and immunohistochemistry, are often invasive, time-consuming, and limited in sensitivity, highlighting the demand for non-invasive, highly sensitive detection methods. In this study, we fabricated a cerium oxide (CeO₂) and graphene oxide (GO) nanocomposite-based aptasensor for the non-invasive detection of EGFR using electrochemical impedance spectroscopy (EIS). The CeO₂-GO nanocomposite was synthesized via the sol-gel method and characterized through UV–Vis spectroscopy, FTIR, TEM, and XRD, confirming the crystalline structure of hexagonal CeO₂ nanoparticles on amorphous GO sheets. The nanocomposite was functionalized with aptamers specific to EGFR using covalent coupling reactions. The EIS analysis of the fabricated aptasensor (GCE/CeO₂-GO/EGFR-Apt/BSA) demonstrated a wide linear detection range from 10 fg mL-1 to 100 ng mL-1, with an ultralow detection limit of 1.87 fg mL-1 in PBS, 3.16 fg mL-1 in serum, 5.31 fg mL-1 in sweat, and 6.14 fg mL-1 in saliva samples. These results highlight the aptasensor's high sensitivity, specificity, and potential for real-time, non-invasive EGFR monitoring in clinical samples such as serum, sweat, and saliva. This approach would facilitate early detection of cancer and personalized diagnostics in point-of-care settings.
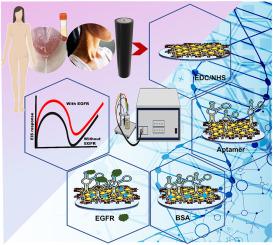
利用铈纳米粒子功能化的氧化石墨烯阻抗传感器对乳腺癌生物标记物进行非侵入式超灵敏检测。
表皮生长因子受体(EGFR)是一种跨膜蛋白,也是与乳腺癌发病机制有关的关键生物标志物。早期精确检测表皮生长因子受体对有效诊断、预后判断和治疗干预至关重要。然而,传统的表皮生长因子受体(EGFR)检测技术,如活检和免疫组化,往往是侵入性的、耗时的,而且灵敏度有限,这凸显了对非侵入性、高灵敏度检测方法的需求。在本研究中,我们制作了一种基于氧化铈(CeO₂)和氧化石墨烯(GO)纳米复合材料的传感器,利用电化学阻抗光谱(EIS)对表皮生长因子受体进行无创检测。通过溶胶-凝胶法合成了 CeO₂-GO 纳米复合材料,并通过紫外-可见光谱、傅立叶变换红外光谱、TEM 和 XRD 对其进行了表征。该纳米复合材料通过共价偶联反应与表皮生长因子受体特异性的适配体功能化。对制备的适配传感器(GCE/CeO₂-GO/EGFR-Apt/BSA)进行的 EIS 分析表明,其线性检测范围从 10 fg mL-1 到 100 ng mL-1,在 PBS、血清、汗液和唾液样品中的超低检测限分别为 1.87 fg mL-1、3.16 fg mL-1、5.31 fg mL-1 和 6.14 fg mL-1。这些结果凸显了该适配传感器的高灵敏度和特异性,以及在血清、汗液和唾液等临床样本中进行实时、无创表皮生长因子受体监测的潜力。这种方法将有助于癌症的早期检测和医疗点的个性化诊断。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biosensors and Bioelectronics
工程技术-电化学
CiteScore
20.80
自引率
7.10%
发文量
1006
审稿时长
29 days
期刊介绍:
Biosensors & Bioelectronics, along with its open access companion journal Biosensors & Bioelectronics: X, is the leading international publication in the field of biosensors and bioelectronics. It covers research, design, development, and application of biosensors, which are analytical devices incorporating biological materials with physicochemical transducers. These devices, including sensors, DNA chips, electronic noses, and lab-on-a-chip, produce digital signals proportional to specific analytes. Examples include immunosensors and enzyme-based biosensors, applied in various fields such as medicine, environmental monitoring, and food industry. The journal also focuses on molecular and supramolecular structures for enhancing device performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


