HSD3B1, prostate cancer mortality and modifiable outcomes
IF 14.6
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
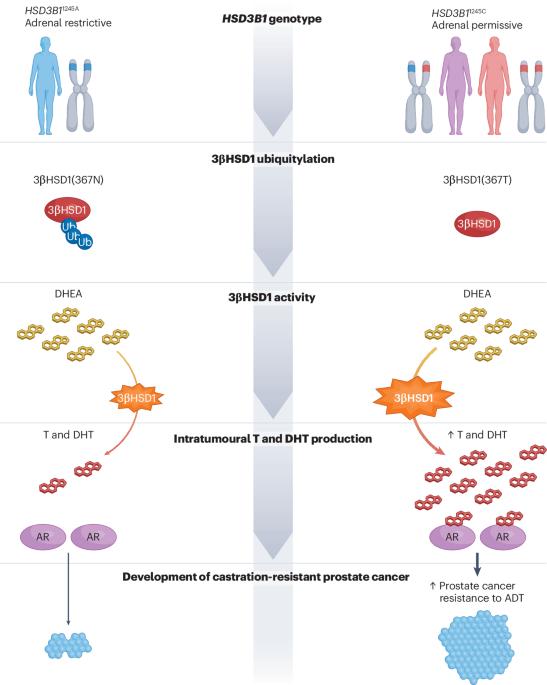
Androgen receptor stimulation by testosterone and dihydrotestosterone is crucial for prostate cancer progression. Despite the initial effectiveness of androgen deprivation therapy (ADT), castration-resistant prostate cancer eventually develops in most men. A common germline missense-encoding polymorphism in HSD3B1 increases extra-gonadal androgen biosynthesis from adrenal precursors owing to increased availability of the encoded enzyme 3β-hydroxysteroid dehydrogenase 1 (3βHSD1) — hence, it is called the adrenal-permissive enzyme. This mechanism explains the more rapid progression to castration-resistant prostate cancer in men who inherit this allele than in men without it via sustained androgen receptor activation despite ADT. Multiple clinical studies, including data derived from prospective phase III studies, have linked adrenal-permissive allele inheritance to inferior clinical responses to ADT and increased mortality, but reversal is possible with upfront adrenal androgen blockade. The adrenal-permissive allele exhibits divergent frequencies across various groups worldwide, which could contribute to differences in clinical outcomes among these populations. Large-scale data from the Million Veteran Program have shown homozygous HSD3B1 adrenal-permissive allele inheritance to be an independent biomarker of prostate cancer-specific mortality. Together, these observations support the integration of HSD3B1 into germline testing and clinical trials as it might help to identify groups at increased likelihood of benefiting from early, intensified, AR-targeting interventions. Lastly, 3βHSD1 is a promising target for pharmacological inhibition, which enables new strategies for systemic prostate cancer therapy. A common germline missense-encoding polymorphism in HSD3B1, called the adrenal-permissive allele, facilitates androgen synthesis from adrenal precursors via increased 3β-hydroxysteroid dehydrogenase 1 (3βHSD1). Here, the authors focus on mechanistic and clinical investigations of 3βHSD1 and the clinical consequences and potential utility of detecting adrenal-permissive allele inheritance.
HSD3B1、前列腺癌死亡率和可改变的结果
睾酮和双氢睾酮对雄激素受体的刺激对前列腺癌的发展至关重要。尽管雄激素剥夺疗法(ADT)最初很有效,但大多数男性最终都会患上对阉割有抵抗力的前列腺癌。HSD3B1 中常见的种系错义编码多态性增加了肾上腺前体对性腺外雄激素的生物合成,这是因为编码酶 3β- 羟基类固醇脱氢酶 1 (3βHSD1) 的可用性增加--因此它被称为肾上腺许可酶。这一机制解释了为什么遗传了这一等位基因的男性比没有遗传这一等位基因的男性更快地发展为耐阉割性前列腺癌,因为尽管有 ADT,但雄激素受体仍会持续激活。包括前瞻性 III 期研究数据在内的多项临床研究表明,肾上腺皮质激素允许性等位基因遗传与 ADT 的临床反应较差和死亡率增加有关,但通过前期肾上腺皮质激素阻断是可以逆转的。肾上腺允许性等位基因在全球不同群体中的频率各不相同,这可能导致这些人群的临床结果存在差异。来自 "百万退伍军人计划"(Million Veteran Program)的大规模数据显示,同源 HSD3B1 肾上腺允许等位基因遗传是前列腺癌特异性死亡率的独立生物标志物。这些观察结果都支持将 HSD3B1 纳入种系检测和临床试验,因为它可能有助于确定更有可能从早期、强化的 AR 靶向干预中获益的群体。最后,3βHSD1 是一个很有希望的药理抑制靶点,可为前列腺癌的系统治疗提供新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Urology
医学-泌尿学与肾脏学
CiteScore
12.50
自引率
2.60%
发文量
123
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Urology is part of the Nature Reviews portfolio of journals.Nature Reviews' basic, translational and clinical content is written by internationally renowned basic and clinical academics and researchers. This journal targeted readers in the biological and medical sciences, from the postgraduate level upwards, aiming to be accessible to professionals in any biological or medical discipline.
The journal features authoritative In-depth Reviews providing up-to-date information on topics within a field's history and development. Perspectives, News & Views articles, and the Research Highlights section offer topical discussions and opinions, filtering primary research from various medical journals.
Covering a wide range of subjects, including andrology, urologic oncology, and imaging, Nature Reviews provides valuable insights for practitioners, researchers, and academics within urology and related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


