Synthesis of Pyrrolidine-2-ylidenes from Isoxazolines and Allenes
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A diastereoselective addition and rearrangement reaction has been developed for the synthesis of pyrrolidine-2-ylidenes from NH-isoxazolines and electron-deficient allenes. This method proceeds via the rearrangement of a proposed N-alkenylisoxazoline intermediate to generate densely functionalized pyrrolidine-2-ylidenes under simple catalyst-free conditions that tolerate ketone substituents and install relative stereochemistry at positions 3 and 4 of the heterocycle. Reaction optimization and the substrate scope are described in addition to studies evaluating the reactivity of the gem-dione and enaminone groups of the products.
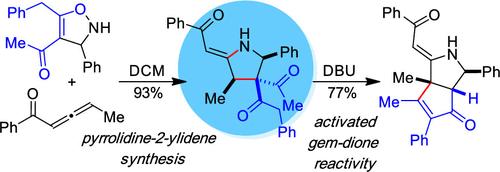
从异噁唑啉类和烯类合成吡咯烷-2-亚基化合物
我们开发了一种非对映选择性加成和重排反应,用于从 NH-异噁唑啉和缺电子异烯合成吡咯烷-2-亚基。该方法是在简单的无催化剂条件下,通过拟议的 N-烯基异噁唑啉中间体的重排反应生成致密官能化的吡咯烷-2-亚基,该反应可容忍酮取代基并在杂环的第 3 位和第 4 位安装相对立体化学结构。除了对产品中的宝石二酮和烯酮基团的反应性进行评估研究外,还介绍了反应优化和底物范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


