Gold(I)-Catalyzed Synthesis of 2,2′-Biindoles via One-Pot Double Cycloisomerization Strategy
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The first systematic, concise and target-directed gold(I)-catalyzed synthesis of a family of 2,2′-biindoles containing different substitution patterns is described. The developed protocol involves the synthesis of 1,3-diyne-anilines followed by a one-pot gold(I)-catalyzed double cycloisomerization, giving rise to an efficient, broad and general protocol to get different 2,2′-biindoles under mild reaction conditions. Due to the methodological restriction of present methods for accessing this class of compounds, herein we present our synthetic proposal which allowed the preparation of several examples of 2,2′-biindoles. Their functionalization-guided us to the discovery that the chemical stability, is substitution structure-dependent.
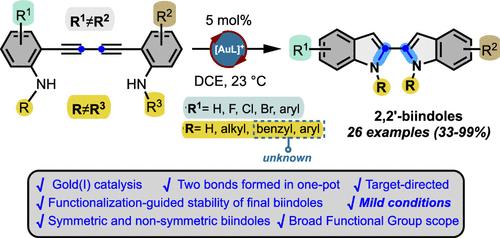
通过一锅双环异构化策略催化金(I)合成 2,2′-双吲哚
本研究首次系统、简明、靶向地描述了由金(I)催化合成含有不同取代模式的 2,2′-双吲哚家族。所开发的方案包括合成 1,3-二炔-苯胺,然后进行一锅金(I)催化的双环异构化,从而产生了一种高效、广泛和通用的方案,可在温和的反应条件下获得不同的 2,2′-双吲哚。由于目前获取这类化合物的方法存在局限性,我们在此介绍我们的合成方案,该方案可以制备多种 2,2′-双吲哚。在对它们进行官能化处理的过程中,我们发现它们的化学稳定性与取代结构有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


