Cu-Catalyzed Multicomponent Reaction of Arylhydrazines with β-Ketoesters and TBN: One-Pot Access to N2-Aryl 1,2,3-Triazole-1-Oxides
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a copper-catalyzed one-pot, multicomponent strategy for the convenient synthesis of N2-aryl 1,2,3-triazole-1-oxides using arylhydrazines, β-ketoesters, and tert-butyl nitrite. This mild and simple reaction proceeds in an atom-economic manner with broad substrate scope, affording a variety of N2-aryl 1,2,3-triazole-1-oxide derivatives. Other salient features of the reaction are good functional group tolerance, scalability, and product diversification.
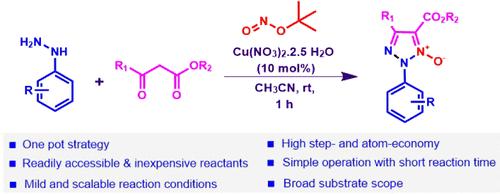
铜催化的芳基肼与β-酮酯和 TBN 的多组分反应:一锅获得 N2-芳基 1,2,3-三唑-1-氧化物
我们报告了一种铜催化的单锅多组分策略,利用芳基肼、β-酮酯和亚硝酸叔丁酯方便地合成 N2 芳基 1,2,3-三唑-1-氧化物。该反应温和简单,以原子经济的方式进行,底物范围广,可得到多种 N2-芳基 1,2,3-三唑-1-氧化物衍生物。该反应的其他突出特点还包括良好的官能团耐受性、可扩展性和产品多样性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


