Efficient Cr(III) removal from effluent using functionalized Ficus benghalensis and Bambusa vulgaris bio-adsorbents
IF 4.1
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Chromium contamination from tannery wastewater poses a significant threat to both the environment and public health, making its removal from effluents crucial before discharge into surface water. This study explores the effectiveness of activated bio-adsorbents made from cost-effective and sustainable sawmill waste Ficus benghalensis (banyan) bark and Bambusa vulgaris (bamboo) for removing Cr (III) from tannery wastewater. The easy accessibility and unique porous structure of these biomaterials make them strong candidates for Cr (III) pollution mitigation. Through carbonization and surface-functionalization via single and two-step methods, the single-step synthesized activated bio-adsorbent exhibited a superior chromium removal rate, achieving 94 % for activated carbon derived from banyan bark (ACBB), compared to 66 % for the two-step process (ACBB*). Single-step synthesis was chosen due to shorter carbonization time, practically viable, and better surface performance of materials. A similar pattern was observed for bamboo-derived activated carbon (ACB), with the one-step process yielding an 82 % removal rate, in contrast to 43 % for the two-step process (ACB*). The bio-absorbents were characterized using XRD, FTIR, BET, SEM, TGA, and zeta potential analysis. Batch process optimization reveals that ACBB and ACB demonstrate maximum chromium removal efficiencies of 92 % and 76 %, respectively, under specific pH, dosage, and temperature conditions. The adsorbents adhere to the Freundlich isotherm model, indicating multilayer adsorption, and follow pseudo-second-order kinetics, suggesting chemisorption. The maximum adsorption capacities were 278.44 mg/g for ACBB and 212.99 mg/g for ACB, which are considerably higher than the findings of previous researchers for similar materials. Thermodynamic analysis reveals that Cr (III) adsorption is spontaneous and endothermic for ACBB, while it is spontaneous and exothermic for ACB. Furthermore, the adsorbents maintain efficacy over three regeneration cycles with less than a 10 % reduction in performance. This research can facilitate efficient Cr (III) removal in industrial-scale tannery wastewater treatment due to its reusability and high adsorption performance, while also guiding future investigations. The potential of these eco-friendly bio-adsorbents for scalable, industrial tannery wastewater treatment offers a sustainable solution for chromium removal.
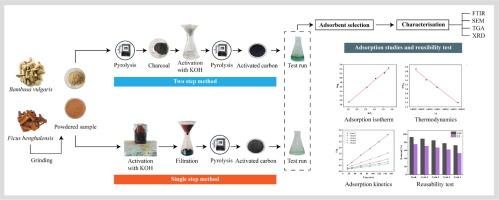

利用功能化榕树和簕杜鹃生物吸附剂高效去除污水中的 Cr(III)
制革废水中的铬污染对环境和公众健康都构成了严重威胁,因此,在将铬排放到地表水之前将其从废水中去除至关重要。本研究探讨了用具有成本效益和可持续性的锯木厂废料榕树皮和竹子制成的活性生物吸附剂去除制革废水中铬(III)的有效性。这些生物材料的易获取性和独特的多孔结构使其成为缓解铬(III)污染的有力候选材料。通过单步法和两步法进行碳化和表面功能化,单步法合成的活性生物吸附剂表现出卓越的铬去除率,榕树皮活性炭(ACBB)的铬去除率达到 94%,而两步法(ACBB*)的铬去除率为 66%。之所以选择单步合成法,是因为碳化时间更短、实际可行,而且材料的表面性能更好。竹制活性炭(ACB)的情况也类似,一步法工艺的去除率为 82%,而两步法工艺(ACB*)的去除率为 43%。利用 XRD、FTIR、BET、SEM、TGA 和 zeta 电位分析对生物吸附剂进行了表征。批量工艺优化结果表明,在特定的 pH 值、用量和温度条件下,ACBB 和 ACB 的最大铬去除率分别为 92 % 和 76 %。这两种吸附剂符合 Freundlich 等温线模型,表明它们具有多层吸附作用,并遵循假二阶动力学,表明它们具有化学吸附作用。ACBB 和 ACB 的最大吸附容量分别为 278.44 毫克/克和 212.99 毫克/克,大大高于之前研究人员对类似材料的研究结果。热力学分析表明,ACBB 对 Cr (III) 的吸附是自发的、内热的,而 ACB 则是自发的、放热的。此外,这些吸附剂在三个再生周期中都能保持功效,性能降低不到 10%。由于其可重复使用性和高吸附性能,这项研究有助于在工业规模的制革废水处理中高效去除 Cr (III),同时也为未来的研究提供了指导。这些生态友好型生物吸附剂在可扩展的工业制革废水处理中的潜力为铬的去除提供了一种可持续的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


