Efficient green separation of Fe-Mn magnetics in manganese sulfate residue via Mn morphology control
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The long-term accumulation of manganese sulfate residues can lead to the release of soluble manganese ions, thereby contaminating the surrounding environment. Although numerous methods exist to address the issue of soluble manganese ions, current harmless treatment approaches fail to consider the subsequent utilization of manganese slag. This study aims to control the magnetic properties of processed residues by adjusting the addition of barium hydroxide and the reaction duration. Our results reveal that the harmless treatment of manganese sulfate slag is achievable with various amounts of barium hydroxide, and the magnetic properties of the reconstructed slag increase with the increased barium hydroxide dosage. Magnetic separation is employed to separate the magnetic components from the reconstructed slag, and the distribution state of these magnetic substance is modulated by altering the particle size and reagent addition. Under optimal conditions, this study finds that the grade of iron was 24.17 %, the grade of manganese was 8.08 %, and the recovery could reach 79 % for iron and 70 % for manganese. Finally, the fining slag obtained by magnetic separation was utilized as an additive in the preparation of ferrites, reducing the minimum reflection loss of the ferrites to −16.18 dB, signifying up to 90 % electromagnetic wave attenuation. These findings indicate that the fining slags holds great potential as a dopant for ferrites, offering a viable pathway for the resourceful and environmentally friendly treatment of manganese sulfate residues.
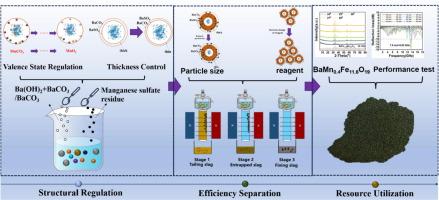

通过锰形态控制实现硫酸锰残渣中铁锰磁性的高效绿色分离
硫酸锰残渣的长期积累会导致可溶性锰离子的释放,从而污染周围环境。虽然有许多方法可以解决可溶性锰离子的问题,但目前的无害化处理方法没有考虑到锰渣的后续利用。本研究旨在通过调整氢氧化钡的添加量和反应时间来控制处理后渣料的磁性能。我们的研究结果表明,使用不同量的氢氧化钡可以实现硫酸锰渣的无害化处理,并且重建渣的磁性能随着氢氧化钡用量的增加而提高。采用磁分离技术从重构炉渣中分离出磁性成分,并通过改变粒度和试剂添加量来调节这些磁性物质的分布状态。研究发现,在最佳条件下,铁的品位为 24.17%,锰的品位为 8.08%,铁的回收率可达 79%,锰的回收率可达 70%。最后,通过磁选获得的细化矿渣被用作制备铁氧体的添加剂,使铁氧体的最小反射损耗降低到 -16.18 dB,电磁波衰减高达 90%。这些研究结果表明,细化渣作为铁氧体的掺杂剂具有巨大的潜力,为资源化和环境友好型处理硫酸锰残渣提供了一条可行的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


