The n–π* electronic transition induced by nitrogen vacancies enhances photocatalytic hydrogen production in carbon nitride
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In semiconductor catalysts, long-lived excited states can effectually improve the utilization of photogenerated carriers to enhance photocatalytic performance. Herein, we used supramolecular engineering to synthesize a hollow tubular carbon nitride catalyst with N vacancies and an obvious n–π* transition. The unique hollow tubular structure provides abundant active sites, which are favorable for photocatalytic reaction. The presence of N vacancies expands the π-electron delocalization domains in the conjugated system, which excites the n–π* transition and thus triggers the red-shifted absorption edge at approximately 660 nm. Experiments and DFT calculations demonstrated that the N vacancies are beneficial for narrowing the bandgap and promoting the reduction of H+ by photogenerated electrons. Femtosecond transient absorption spectroscopy (fs-TAS) indicated that the n–π* electronic transition in the carbon nitride photocatalyst leads to slower exciton annihilation (lifetime: 38.64 ± 10.6 ps) and extended shallow electron trapping states (lifetime: 325.9 ± 19.3 ps). The appearance of these states adds more photogenerated electrons to the photocatalytic reaction process. The optimal hollow tubular carbon nitride catalyst exhibits a hydrogen production rate of 2664.47 μmol∙g−1∙h−1, which is 31.2 times higher than that of bulk carbon nitride (85.3325 μmol∙g−1∙h−1). This work highlights the ability of the n–π* transition induced by N vacancies to enhance the photocatalytic activity of carbon nitride.
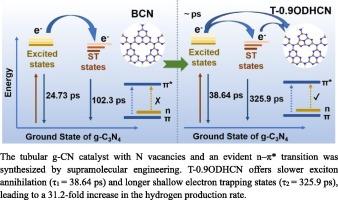
氮空位诱导的 n-π* 电子转变增强了氮化碳的光催化制氢能力
在半导体催化剂中,长寿命激发态可有效提高光生载流子的利用率,从而增强光催化性能。在此,我们利用超分子工程合成了一种具有 N 空位和明显 n-π* 转变的空心管状氮化碳催化剂。独特的空心管状结构提供了丰富的活性位点,有利于光催化反应。N 空位的存在扩大了共轭体系中的π电子析出域,激发了 n-π* 转变,从而引发了约 660 纳米波长处的红移吸收边。实验和 DFT 计算表明,N 空位有利于缩小带隙,促进光生电子还原 H+。飞秒瞬态吸收光谱(fs-TAS)表明,氮化碳光催化剂中的 n-π* 电子转变会导致更慢的激子湮灭(寿命:38.64 ± 10.6 ps)和更长的浅电子捕获态(寿命:325.9 ± 19.3 ps)。这些态的出现为光催化反应过程增加了更多的光生电子。最佳空心管状氮化碳催化剂的制氢率为 2664.47 μmol∙g-1∙h-1,是块状氮化碳催化剂(85.3325 μmol∙g-1∙h-1)的 31.2 倍。这项工作凸显了氮空位诱导的 n-π* 转变能够提高氮化碳的光催化活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


