In Situ Construction of Bacterial Dressing for Low-Temperature Sterilization in Infected Lesions
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
While photothermal sterilization offers significant advantages over antibiotics for treating surgical site infections, its application is limited by side effects caused by excessive heat. To address this dilemma, this study focuses on the often-overlooked factor of temperature inhomogeneity within irradiated regions, which impacts photothermal efficacy. We propose a solution termed “bacterial photothermal dressing”. In this approach, tannic acid (TA) first attaches to bacteria or biofilm and subsequently captures surrounding FeIII ions to form a close-fitting FeIIITA dressing in situ. For comparison, a conventional photothermal sterilization method is established by directly administering FeIIITA nanoparticles to infected sites. The bacterial photothermal dressing approach allows for bacterial elimination at much lower temperatures, minimizing heat-related side effects. Moreover, the acidic microenvironment within the biofilm can trigger the gradual release of TA, permitting continuous antiseptic and anti-inflammatory effects. This sustained activity enhances antibacterial efficacy and helps prevent secondary infection following photothermal treatment. Consequently, this approach significantly accelerates wound healing by improving bactericidal efficiency and reducing inflammatory responses.
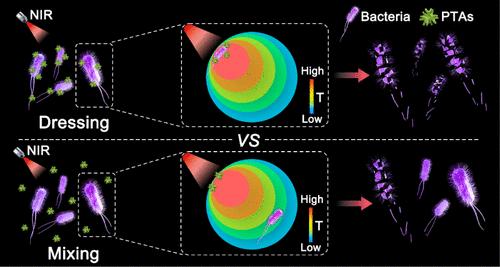
原位构建用于感染病灶低温灭菌的细菌敷料
虽然光热杀菌在治疗手术部位感染方面比抗生素具有显著优势,但其应用却受到过热引起的副作用的限制。为了解决这一难题,本研究重点关注经常被忽视的因素,即照射区域内的温度不均匀性会影响光热疗效。我们提出了一种名为 "细菌光热敷料 "的解决方案。在这种方法中,单宁酸(TA)首先附着在细菌或生物膜上,然后捕获周围的 FeIII 离子,在原位形成贴合的 FeIIITA 敷料。相比之下,传统的光热灭菌方法是通过在感染部位直接施用 FeIIITA 纳米粒子来实现的。细菌光热敷料法可以在更低的温度下消除细菌,最大限度地减少与热有关的副作用。此外,生物膜内的酸性微环境可促使 TA 逐步释放,从而实现持续的杀菌和消炎效果。这种持续的活性增强了抗菌效果,有助于防止光热治疗后的二次感染。因此,这种方法通过提高杀菌效率和减少炎症反应,大大加快了伤口愈合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


