Delithiation-induced secondary phase formation in Li-rich cathode materials
IF 10.7
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Li-rich layered oxides can potentially provide high capacity, thereby enhancing energy density as cathode materials in Li-ion batteries. However, one of the main drawbacks is their low cycling stability. It has been proposed that the structural stability of a solid solution compound might be enhanced by exploiting the high-entropy concept. Here, we studied two Li-rich layered oxide cathode materials with multiple cations in their transition metal sites, categorized as medium or high entropy: Li(Li0.2Co0.18Ni0.18Mn0.44)O2 and Li(Li0.2Co0.18Ni0.18Mn0.18Ti0.26)O2. The synthesized materials, however, experienced a large capacity loss during the first charge/discharge cycle. We performed first-principles calculations to understand the mechanism behind the capacity fading and discovered significant structural changes in both systems. Specifically, we observed extensive Li/Ni interchange, migration of transition metal ions to Li sites, and formation of secondary phases. For the Ti-containing material, which shows a larger capacity fade than the other system, we even observed the formation of a spinel phase. The computed enthalpies of secondary phase formation reactions exhibit large negative values. However, the estimated (maximum) configurational entropy contributions to the free energies of these reactions are much smaller and therefore not determining factors. This study provides crucial insights into degradation mechanisms in Li-rich high-entropy systems, aiding the future design and development of advanced cathode materials for next-generation lithium-ion batteries.
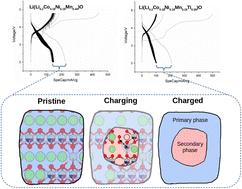
富锂离子阴极材料中的去硫酸盐化诱导的次生相形成
富锂层状氧化物作为锂离子电池的阴极材料,有可能提供高容量,从而提高能量密度。然而,其主要缺点之一是循环稳定性较低。有人提出,可以通过利用高熵概念来增强固溶体化合物的结构稳定性。在此,我们研究了两种富含锂的层状氧化物阴极材料,它们的过渡金属位点中含有多个阳离子,被归类为中熵或高熵:Li(Li0.2Co0.18Ni0.18Mn0.44)O2 and Li(Li0.2Co0.18Ni0.18Mn0.18Ti0.26)O2.然而,合成的材料在第一个充放电周期中出现了较大的容量损失。我们进行了第一原理计算,以了解容量衰减背后的机理,并发现这两种体系都发生了显著的结构变化。具体来说,我们观察到了广泛的锂/镍交换、过渡金属离子向锂位迁移以及次生相的形成。含钛材料的容量衰减比其他体系更大,我们甚至观察到尖晶石相的形成。计算得出的次生相形成反应焓呈现较大的负值。然而,这些反应的自由能的估计(最大)构型熵贡献要小得多,因此不是决定性因素。这项研究为富锂高熵体系的降解机制提供了重要见解,有助于未来新一代锂离子电池先进正极材料的设计和开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


