Hybrid-DFT Molecular Dynamics Simulations of Photocatalytic Water Oxidation in a [Ru-bda]–Dye Complex
IF 4.3
3区 材料科学
Q1 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
Abstract
In the past decade, Ru-bda (bda = 2,2′-bipyridine-6,6′-dicarboxylic acid) complexes have emerged as extremely effective water oxidation catalysts, rendering them a potential candidate for incorporation into dye-sensitized photoelectrochemical cells. However, the performance of these catalysts declines dramatically when anchored to a photoanode surface due to their catalytic mechanism involving the interaction of two metal centers (I2M). This reduced performance prompts an investigation into the catalytic cycle following an alternative mechanism in which the O–O bond is formed through a water nucleophilic attack (WNA). In this work, we have performed hybrid-DFT based molecular dynamics simulations of the rate-determining O–O bond formation following the WNA mechanism in a [Ru-bda]–dye dyad model in explicit water solvation. In addition, our study probes oxygen dissociation from the RuIII–O2 intermediate, and the equilibrium dynamics of the low-valent RuIII–bda intermediate. Our simulations demonstrate that including a fraction of exact Hartree–Fock exchange impacts the electron and hole localizations in the catalyst–dye complex, which can in specific instances affect the dynamics of the system. This study contributes to a fundamental understanding of water oxidation catalysis with the Ru-bda catalyst family and highlights the relevance of modeling catalytic processes at the hybrid-DFT level.
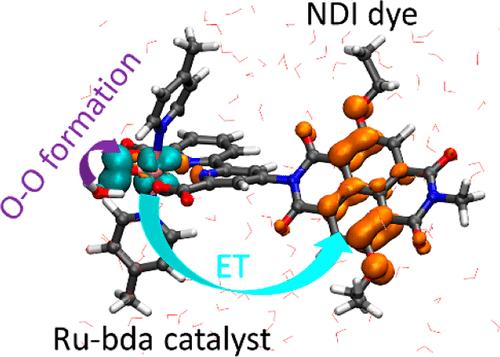
Ru-bda 染料复合物光催化水氧化的混合-DFT 分子动力学模拟
在过去十年中,Ru-bda(bda = 2,2′-联吡啶-6,6′-二羧酸)复合物已成为极其有效的水氧化催化剂,使其成为染料敏化光电化学电池的潜在候选物质。然而,由于其催化机理涉及两个金属中心(I2M)的相互作用,当这些催化剂锚定在光阳极表面时,其性能会急剧下降。性能的下降促使我们研究催化循环的另一种机制,即通过亲水核攻击(WNA)形成 O-O 键。在这项工作中,我们对显式水溶液中[Ru-bda]-染料二元模型中通过 WNA 机制形成决定速率的 O-O 键进行了基于混合-DFT 的分子动力学模拟。此外,我们的研究还探究了 RuIII-O2 中间体的氧解离以及低价 RuIII-bda 中间体的平衡动力学。我们的模拟证明,加入一部分精确的 Hartree-Fock 交换会影响催化剂-染料复合物中的电子和空穴定位,在特定情况下会影响系统的动力学。这项研究有助于从根本上理解 Ru-bda 催化剂家族的水氧化催化作用,并突出了在混合-DFT 水平上模拟催化过程的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


