Mechanism-Based Thermodynamic Analysis for One-Step and Two-Step Ethanol-to-1,3-Butadiene Conversion Processes
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
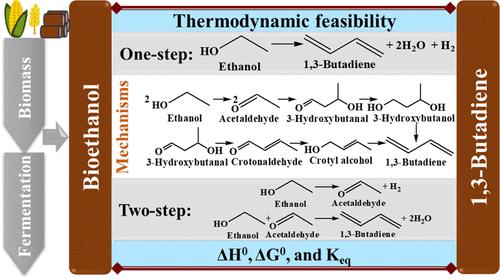
Renewable 1,3-butadiene (BD) is essential for sustainability of the synthetic rubber sector. This work presents a comprehensive thermodynamic analysis for one- and two-step ethanol-to-BD conversion processes. The two-step process comprises ethanol dehydrogenation, followed by the condensation of acetaldehyde with another ethanol molecule into BD. The process involves a complex reaction network with a wide range of byproducts depending on the nature of the catalysts and operating conditions, lacking unique consensus on the C–C bond-forming mechanism. This study elucidates the temperature regime for the spontaneity of the reactions proposed in various mechanisms and side reactions based on the standard Gibbs free energy change. The equilibrium conversion and product selectivity were further calculated under a wide temperature and pressure range. The overall reaction in the one-step process is thermodynamically spontaneous above 417 K, while the first and second steps of the two-step process are spontaneous above 550 and 285 K, respectively. Excepting Prins condensation, other mechanisms lack the spontaneity of all reaction steps. The equilibrium BD selectivity is favorable at elevated temperatures and low pressures. The addition of acetaldehyde in the two-step process has a favorable impact with higher BD selectivity, the maximum being at a 1:1 molar ratio of ethanol/acetaldehyde. This study elucidates thermodynamic insights into existing mechanisms and drives the evolution of a feasible mechanism. This effort will eventually help design novel catalysts and optimized processes for sustainable biobased BD production using ethanol derived from renewable feedstocks, aligning with the global commitment to greener and resource-friendly chemical manufacturing.
基于机理的一步法和两步法乙醇-1,3-丁二烯转化过程的热力学分析
可再生的 1,3-丁二烯(BD)对合成橡胶行业的可持续发展至关重要。本研究对一步法和两步法乙醇-丁二烯转化过程进行了全面的热力学分析。两步法包括乙醇脱氢,然后乙醛与另一个乙醇分子缩合成 BD。该过程涉及一个复杂的反应网络,根据催化剂的性质和操作条件的不同,会产生多种副产物,而 C-C 键的形成机理却缺乏共识。本研究根据标准吉布斯自由能变化,阐明了各种机理和副反应所提出的反应自发温度机制。并进一步计算了在较宽温度和压力范围内的平衡转化率和产物选择性。一步法反应的总反应在 417 K 以上热力学自发,而两步法反应的第一步和第二步分别在 550 K 和 285 K 以上自发。除了普氏缩合之外,其他机制的所有反应步骤都不具有自发性。在高温和低压条件下,平衡 BD 选择性较好。在两步反应过程中加入乙醛对提高 BD 选择性有有利影响,乙醇/乙醛摩尔比为 1:1 时选择性最大。这项研究阐明了现有机制的热力学原理,并推动了可行机制的发展。这项工作最终将有助于设计新型催化剂和优化工艺,利用从可再生原料中提取的乙醇进行可持续的生物基 BD 生产,从而与全球对更环保和资源友好型化学品生产的承诺保持一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


