Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
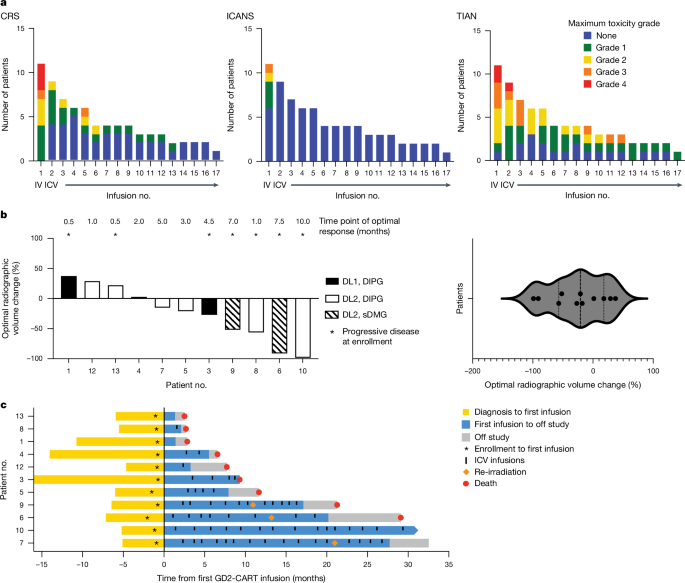
H3K27M-mutant diffuse midline gliomas (DMGs) express high levels of the disialoganglioside GD2 (ref. 1). Chimeric antigen receptor-modified T cells targeting GD2 (GD2-CART) eradicated DMGs in preclinical models1. Arm A of Phase I trial no. NCT04196413 (ref. 2) administered one intravenous (IV) dose of autologous GD2-CART to patients with H3K27M-mutant pontine (DIPG) or spinal DMG (sDMG) at two dose levels (DL1, 1 × 106 kg−1; DL2, 3 × 106 kg−1) following lymphodepleting chemotherapy. Patients with clinical or imaging benefit were eligible for subsequent intracerebroventricular (ICV) intracranial infusions (10–30 × 106 GD2-CART). Primary objectives were manufacturing feasibility, tolerability and the identification of maximally tolerated IV dose. Secondary objectives included preliminary assessments of benefit. Thirteen patients enroled, with 11 receiving IV GD2-CART on study (n = 3 DL1 (3 DIPG); n = 8 DL2 (6 DIPG, 2 sDMG)). GD2-CART manufacture was successful for all patients. No dose-limiting toxicities occurred on DL1, but three patients experienced dose-limiting cytokine release syndrome on DL2, establishing DL1 as the maximally tolerated IV dose. Nine patients received ICV infusions, with no dose-limiting toxicities. All patients exhibited tumour inflammation-associated neurotoxicity, safely managed with intensive monitoring and care. Four patients demonstrated major volumetric tumour reductions (52, 54, 91 and 100%), with a further three patients exhibiting smaller reductions. One patient exhibited a complete response ongoing for over 30 months since enrolment. Nine patients demonstrated neurological benefit, as measured by a protocol-directed clinical improvement score. Sequential IV, followed by ICV GD2-CART, induced tumour regressions and neurological improvements in patients with DIPG and those with sDMG. We evaluated the use of chimeric antigen receptor-modified T cells targeting GD2 (GD2-CART) for H3K27M+ diffuse midline glioma (DMG), finding that intravenous administration of GD2-CART, followed by intracranial infusions, induced tumour regressions and neurological improvements in patients with H3K27M-mutant pontine or spinal DMG.

静脉注射和颅内 GD2-CAR T 细胞治疗 H3K27M+ 弥漫中线胶质瘤
H3K27M突变的弥漫中线胶质瘤(DMGs)表达高水平的二异抗神经胶质苷GD2(参考文献1)。在临床前模型中,靶向 GD2 的嵌合抗原受体修饰 T 细胞(GD2-CART)可根除 DMGs2。第一阶段试验的 A 组(NCT04196413 号试验NCT04196413(参考文献 3)的 I 期试验 A 组在淋巴清除化疗后,以两种剂量水平(DL1,1 × 106 kg-1;DL2,3 × 106 kg-1)给 H3K27M 突变的桥脑(DIPG)或脊髓 DMG(sDMG)患者静脉注射一次自体 GD2-CART。临床或影像学获益的患者有资格接受后续的脑室内(ICV)颅内输注(10-30 × 106 GD2-CART)。首要目标是制造可行性、耐受性和最大耐受静脉注射剂量的确定。次要目标包括效益的初步评估。13名患者参加了研究,其中11人在研究中接受了静脉注射GD2-CART(n = 3 DL1(3 DIPG);n = 8 DL2(6 DIPG,2 sDMG))。所有患者都成功制造了 GD2-CART。DL1没有出现剂量限制性毒性反应,但有3名患者在DL2出现了剂量限制性细胞因子释放综合征,从而确定DL1为最大耐受静脉注射剂量。九名患者接受了 ICV 输注,没有出现剂量限制性毒性反应。所有患者都出现了肿瘤炎症相关的神经毒性,通过强化监测和护理得到了安全控制。四名患者的肿瘤体积大幅缩小(分别为52、54、91和100%),另有三名患者的肿瘤体积缩小幅度较小。一名患者表现出完全应答,自入选以来已持续 30 多个月。根据方案指导的临床改善评分,九名患者显示出神经系统获益。连续静脉注射后再注射ICV GD2-CART,可使DIPG患者和sDMG患者的肿瘤缩小,神经功能得到改善。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


