Unlocking the Production of Biomass-Derived Plastic Monomer 2,5-Furandicarboxylic Acid at Industrial-Level Concentration
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
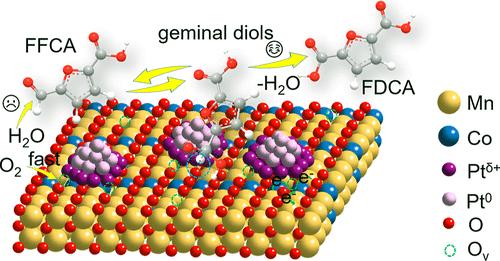
2,5-Furandicarboxylic acid (FDCA) is a promising biomass-derived alternative to fossil-based terephthalic acid. The catalytic oxidation of 5-hydroxymethylfurfural (HMF) to FDCA is widely recognized as a viable route for producing FDCA at industrially relevant concentrations (approximately 20 wt %); however, this has not yet been achieved. Toward this goal, we report that through controlled engineering of an oxygen-vacancy-enriched Mn/Co oxide as the support for Pt nanoparticles, a heterostructure of Pt/PtO2 with electron-rich interfacial Pt–O–Mn sites (Pt/Mn10Co1Ox-VC) is formed, significantly enhancing the adsorption and activation of O2, HMF, and its key intermediates. As a result, selective oxidation of both HMF (up to 40 wt %) and crude HMF (10 wt % and 70 wt % purity) was achieved with high FDCA yields ranging from 83% to 95% under base-free conditions, demonstrating strong economic feasibility and industrial potential for FDCA production. This work highlights the rational design of interfacial structures for the efficient oxidation of biomass-derived aldehydes and alcohols to bio-based dicarboxylic acids at industrially relevant concentrations, paving the way for FDCA to serve as a sustainable alternative to terephthalic acid as a comonomer in polyester production.
以工业水平的浓度生产生物质塑料单体 2,5-呋喃二甲酸
2,5-呋喃二甲酸(FDCA)是一种很有前景的生物质替代化石对苯二甲酸的来源。5-hydroxymethylfurfural (HMF) 催化氧化成 FDCA 被广泛认为是生产工业相关浓度(约 20 wt %)FDCA 的可行途径;然而,这一目标尚未实现。为了实现这一目标,我们报告了通过对作为铂纳米粒子支撑的氧空位富集锰/钴氧化物进行控制工程,形成了具有富电子界面铂-氧-锰位点(Pt/Mn10Co1Ox-VC)的异质结构 Pt/PtO2,显著增强了对 O2、HMF 及其关键中间产物的吸附和活化。因此,在无碱条件下,HMF(纯度高达 40 wt %)和粗 HMF(纯度分别为 10 wt % 和 70 wt %)都实现了选择性氧化,FDCA 产率高达 83% 至 95%,证明了 FDCA 生产具有很强的经济可行性和工业潜力。这项工作强调了界面结构的合理设计,可在工业相关浓度下将生物质衍生醛和醇高效氧化为生物基二羧酸,为 FDCA 在聚酯生产中作为对苯二甲酸的可持续替代共聚单体铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
文献相关原料
公司名称
产品信息
阿拉丁
HMF
阿拉丁
FDCA
阿拉丁
HMFCA
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


