X-ray Reflectivity Probing the Structural Evolution of Sunflower Proteins Adsorbed at the Air–Water Interface
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The study delves into the adsorption of sunflower proteins at the air/water interface using specular X-ray reflection. The research involved fitting models of the protein films to the reflectivity data, resulting in detailed images of the X-ray scattering length density profiles perpendicular to the air/water interface. The sunflower protein isolate that is examined consists of multiple components, and the study proposes a transition from a 1-slab model to a 4-slab model to represent the changing layer structure over time. This transition is significant as it reflects the increasing complexity of the protein film as more proteins adsorb at the interface. Initially, sunflower proteins form a monolayer at the air/water boundary, consisting of a protein-rich, hydrophobic portion closest to the interface and a more diffuse, hydrophilic portion extending into the bulk aqueous phase. The structural changes at the interface over time depend on the bulk protein concentration in the solution. For solutions at relatively low concentrations (C ≤ 0.5 g/L), a lower amount of adsorption results in a larger, more extensive interface area for each species and a thinner protein adsorption layer. The overall thickness of a saturated monolayer is approximately 100 Å, which is close to the maximum dimension of sunflower globulins, with the thickness of the corresponding hydrophobic portion being about 20 Å. For solutions at relatively high concentrations (C ≥ 1.0 g/L), even after forming a saturated monolayer, structural evolution continues within the experimental time frame, occurring on both hydrophilic and hydrophobic sides. Additional proteins from the bulk diffuse toward the interface, forming an extra layer in the water phase and causing an increase in the overall thickness. Furthermore, a distinct sublayer develops next to the air phase, indicating a further structuration of the hydrophobic portion.
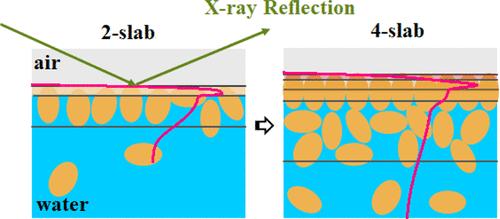
X 射线反射探测吸附在空气-水界面上的向日葵蛋白质的结构演变
该研究利用镜面 X 射线反射深入研究了向日葵蛋白质在空气/水界面的吸附情况。研究工作包括根据反射率数据拟合蛋白质薄膜的模型,从而得到垂直于空气/水界面的 X 射线散射长度密度剖面的详细图像。所研究的向日葵分离蛋白由多种成分组成,研究提出了从单层模型到四层模型的过渡,以表示随时间变化的层结构。这种过渡非常重要,因为它反映了随着更多蛋白质吸附在界面上,蛋白质膜的复杂性不断增加。最初,向日葵蛋白质在空气/水边界形成一个单层,由最靠近界面的富含蛋白质的疏水部分和延伸到大量水相中的扩散性更强的亲水部分组成。随着时间的推移,界面上的结构变化取决于溶液中的大量蛋白质浓度。对于浓度相对较低(C ≤ 0.5 g/L)的溶液,较低的吸附量会导致每个物种的界面面积更大、更广,蛋白质吸附层更薄。饱和单层的总厚度约为 100 Å,接近向日葵球蛋白的最大尺寸,相应疏水部分的厚度约为 20 Å。对于浓度相对较高的溶液(C ≥ 1.0 g/L),即使在形成饱和单层后,结构演变仍会在实验时间范围内继续,亲水和疏水两侧都会发生。大量蛋白质向界面扩散,在水相中形成一个额外的层,导致整体厚度增加。此外,在气相旁边还出现了一个明显的亚层,表明疏水部分的结构发生了进一步的变化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


