Rigid shell and flexible pore for highly efficient and reversible sulfur-carbon co-adsorption by hierarchical porous Mg-gallate@Poly(acrylate)
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The flue gas from coal combustion emits mass CO2 and trace SO2, which are harmful to the environment. To address the aforementioned issues, the development of a high-performance sulfur-carbon co-adsorption material is essential for the simultaneous capture of CO2 and SO2. A special hierarchical pore structure of Mg-gallate@Poly(acrylate) is synthesized here by self-nanocrystallization flexible metal–organic frameworks (MOFs) load a rigid and hydrophobic polymer macroporous surface. Adsorption results indicate that this material exhibits more pronounced characteristics, with an equivalent CO2 adsorption capacity to Mg-gallate powder and a 25 % increase in SO2 adsorption capacity. Dynamic gas adsorption data show that Mg-gallate@Poly(acrylate) has a fast adsorption rate and easy regeneration. Moreover, the adsorption performance of Mg-gallate@Poly(acrylate) in 2000 ppm SO2 slightly rises to that in a non-sulfur environment. The adsorption capacity of CO2 and SO2 can reach 76.5 and 45 mL/g, respectively. The reason for the sulfur-carbon co-adsorption of this material is further demonstrated by theoretical calculations. Additionally, Mg-gallate@Poly(acrylate) shows excellent hydrothermal stability and cyclic regenerability of SO2 and CO2. Thus, these results indicate that Mg-gallate@Poly(acrylate) satisfies the process requirements for sulfur-carbon co-adsorption.
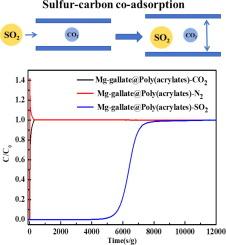
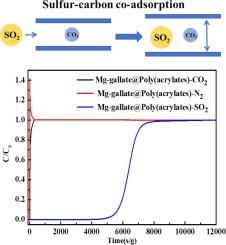
通过分层多孔 Mg-gallate@Poly(丙烯酸酯)实现刚性外壳和柔性孔隙对硫-碳的高效、可逆共吸附
燃煤产生的烟气会排放大量二氧化碳和微量二氧化硫,对环境造成危害。为了解决上述问题,开发一种高性能的硫碳共吸附材料对于同时捕集二氧化碳和二氧化硫至关重要。本文通过自纳米结晶柔性金属有机框架(MOFs)负载刚性疏水聚合物大孔表面,合成了一种特殊的分层孔结构 Mg-gallate@Poly(acrylate)。吸附结果表明,这种材料具有更明显的特性,其二氧化碳吸附能力与 Mg-gallate 粉末相当,二氧化硫吸附能力提高了 25%。动态气体吸附数据表明,Mg-gallate@Poly(丙烯酸酯)具有吸附速度快、易于再生的特点。此外,Mg-gallate@Poly(丙烯酸酯)在 2000 ppm 二氧化硫环境中的吸附性能略高于在无硫环境中的吸附性能。CO2 和 SO2 的吸附容量分别达到 76.5 和 45 mL/g。理论计算进一步证明了这种材料硫碳共吸附的原因。此外,Mg-gallate@Poly(丙烯酸酯)还表现出优异的水热稳定性和对 SO2 和 CO2 的循环再生能力。因此,这些结果表明,Mg-gallate@Poly(丙烯酸酯)符合硫碳共吸附的工艺要求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


