Different paths, same destination: Bisphenol A and its substitute induce the conjugative transfer of antibiotic resistance genes
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Antibiotic resistance genes are primarily spread through horizontal gene transfer in aquatic environments. Bisphenols, which are widely used in industry, are pervasive contaminants in such environments. This study investigated how environmentally relevant concentrations of bisphenol A and its substitute (bisphenol S, Bisphenol AP and Bisphenol AF) affect the spread of antibiotic resistance genes among Escherichia coli. As a result, bisphenol A and its three substitutes were found to promote the RP4 plasmid-mediated conjugative transfer of antibiotic resistance genes with different promotive efficiency. Particularly, bisphenol A and bisphenol S were found to induce more than double the incidence of conjugation at 0.1 nmol/L concentration. They therefore were selected as model compounds to investigate the involved mechanisms. Surprisingly, both slightly inhibited bacterial activity, but there was no significant increase in cell death. Bisphenols exposure changed the polymeric substances excreted by the bacteria, increased the permeability of their cell membranes, induced the secretion of antioxidant enzymes and generated reactive oxygen species. They also affected the expression of genes related to conjugative transfer by upregulating replication and DNA transfer genes and downregulating global regulatory genes. It should be noted that gene expression levels were higher in the BPS-exposed group than in the BPA-exposed group. The synthesis of bacterial metabolites and functional components was also significantly affected by bisphenols exposure. This research has helped to clarify the potential health risks of bisphenol contamination of aquatic environments.
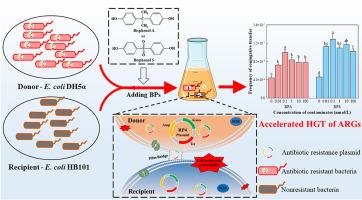
殊途同归双酚 A 及其替代品诱导抗生素抗性基因的共轭转移。
抗生素耐药性基因主要通过水生环境中的水平基因转移传播。在工业中广泛使用的双酚是此类环境中普遍存在的污染物。本研究调查了环境相关浓度的双酚 A 及其替代品(双酚 S、双酚 AP 和双酚 AF)如何影响抗生素耐药基因在大肠杆菌中的传播。结果发现,双酚 A 及其三种替代物能促进 RP4 质粒介导的抗生素抗性基因的共轭转移,其促进效率各不相同。特别是双酚 A 和双酚 S,在 0.1 nmol/L 浓度下,它们诱导的共轭发生率是原来的两倍多。因此,我们选择这两种化合物作为模型化合物来研究其中的机制。令人惊讶的是,这两种化合物都能轻微抑制细菌的活性,但细胞死亡并没有显著增加。接触双酚会改变细菌排泄的聚合物质,增加其细胞膜的渗透性,诱导抗氧化酶的分泌,并产生活性氧。它们还通过上调复制和 DNA 转移基因以及下调全局调控基因,影响与共轭转移相关的基因表达。值得注意的是,暴露于 BPS 组的基因表达水平高于暴露于 BPA 组。细菌代谢物和功能成分的合成也受到双酚暴露的显著影响。这项研究有助于澄清双酚污染水生环境对健康的潜在危害。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


