An ultrasound-activated piezoelectric sonosensitizer enhances mitochondrial depolarization for effective treatment of orthotopic glioma
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Despite the significant advancements in piezoelectric materials for sonodynamic therapy (SDT), the suppression of orthotopic glioma remains challenging, primarily due to the unclear mechanism and the restriction of blood-brain barrier (BBB). Herein, we proposed that layered piezoelectric SrBi2Ta2O9 nanoparticles (SBTO NPs) could effectively depolarize the mitochondrial membrane potential (ΔΨm) of glioma cells under ultrasound (US) exposure. The US-induced band bending in SBTO NPs enhanced redox ability, promoting an increase in reactive oxygen species (ROS) generation. The in vitro results proved that SBTO NPs selectively accumulated in mitochondria under US and induced apoptosis in a mitochondrial depolarization manner mediated by the generation of ROS and free charges. Furthermore, SBTO NPs could cross the BBB and then accumulate in gliomas through US/microbubbles (MBs) procedure and protein-mediated transport. The therapeutic effect of piezoelectric SBTO NPs mediated SDT was proved in the orthotopic glioma mouse model. As validated by the histopathological observation and the long-term evaluation, the good biocompatibility and biosafety of SBTO NPs make it possible for deep tumor therapy, and worthy for further preclinical study.
Statement of significance
Employing piezoelectric sonosensitizers for sonodynamic therapy (SDT) has emerged as a promising strategy for cancer treatment; however, the unclear mechanism and blood-brain barrier (BBB) limit the effectiveness of SDT in glioma. Herein, we developed piezoelectric SrBi2Ta2O9 nanoparticles (SBTO NPs) with a built-in electric field for glioma treatment and explored the underlying therapeutic mechanism. Notably, SBTO NPs selectively accumulated in mitochondria under ultrasound (US) and induced apoptosis in a mitochondrial depolarization manner, which is mediated by the generation of reactive oxygen species (ROS) and free charges. In an orthotopic glioma mouse model, SBTO NPs were delivered into the glioma through US/microbubbles and transferrin-mediated transport pathways, inhibiting tumor growth. This work provides a new paradigm for the treatment of orthotopic glioma and other tumor types.
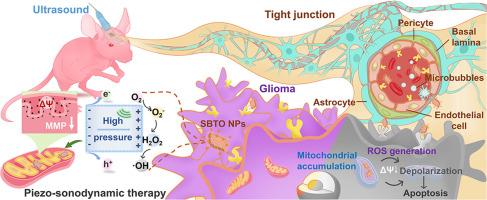
超声激活的压电声纳敏化剂可增强线粒体去极化,从而有效治疗正位胶质瘤。
尽管用于声动力疗法(SDT)的压电材料取得了重大进展,但抑制正位胶质瘤仍具有挑战性,这主要是由于机制不清和血脑屏障(BBB)的限制。在此,我们提出层状压电 SrBi2Ta2O9 纳米粒子(SBTO NPs)可在超声(US)暴露下有效地使胶质瘤细胞的线粒体膜电位(ΔΨm)去极化。US 诱导的 SBTO NPs 带弯曲增强了氧化还原能力,促进了活性氧(ROS)生成的增加。体外研究结果证明,在 US 诱导下,SBTO NPs 可选择性地在线粒体中积聚,并以线粒体去极化的方式通过产生 ROS 和自由电荷诱导细胞凋亡。此外,SBTO NPs 还能穿过 BBB,然后通过 US/微气泡(MBs)程序和蛋白质介导的运输在胶质瘤中聚集。压电式 SBTO NPs 介导的 SDT 治疗效果已在正位胶质瘤小鼠模型中得到证实。组织病理学观察和长期评估证实,SBTO NPs 具有良好的生物相容性和生物安全性,可用于深部肿瘤治疗,值得进一步开展临床前研究。意义声明:利用压电声敏剂进行声动力疗法(SDT)已成为一种很有前景的癌症治疗策略;然而,机制不清和血脑屏障(BBB)限制了 SDT 在胶质瘤中的有效性。在此,我们开发了内置电场的压电 SrBi2Ta2O9 纳米粒子(SBTO NPs)用于胶质瘤治疗,并探索了其潜在的治疗机制。值得注意的是,在超声(US)作用下,SBTO NPs 可选择性地在线粒体中聚集,并以线粒体去极化的方式诱导细胞凋亡,而线粒体去极化是由活性氧(ROS)和自由电荷的产生介导的。在正位胶质瘤小鼠模型中,SBTO NPs 通过 US/微气泡和转铁蛋白介导的运输途径被送入胶质瘤,抑制了肿瘤的生长。这项工作为治疗正位胶质瘤和其他类型的肿瘤提供了新的范例。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


