SUMOylation of TP53INP1 is involved in miR-30a-5p-regulated heart senescence
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Heart senescence is critical for cardiac function. This study aimed to characterize the role and mechanism of action of miR-30a-5p in cardiac senescence. miR-30a-5p was downregulated in aged mouse hearts and neonatal rat cardiomyocytes (NRCMs). In vivo, using a combination of echocardiography and different molecular biological approaches, we investigated the role of miR-30a-5p knockout or overexpression in natural- or D-galactose-induced heart aging in mice. In vitro, using RNA sequencing and a series of molecular biology methods, the mechanism by which miR-30a-5p regulates cardiac senescence was explored in cardiomyocytes. miR-30a-5p knockout mice showed aggravated natural- or D-galactose-induced heart aging compared to wild-type littermate mice, with significantly decreased heart function, an increased number of γH2AX-positive cells, reduced telomere length, and upregulated p21 and p53 expression. Cardiac-specific knockdown of miR-30a-5p using adeno-associated virus 9 in D-galactose-induced senescent wild-type mice resulted in effects similar to those observed in knockout mice. Notably, the overexpression of miR-30a-5p in wild-type murine hearts alleviated D-galactose-induced heart senescence by improving heart function, increasing telomere length, decreasing the number of γH2AX-positive cells, and downregulating p53 and p21 expression. This was confirmed in D-galactose-treated or naturally aged NRCMs. Mechanistically, TP53INP1 was identified as a target of miR-30a-5p by mediating the SUMOylation of TP53INP1 and its translocation from the cytoplasm to the nucleus to interact with p53. Furthermore, this study demonstrated that cardiac-specific TP53INP1 deficiency ameliorates miR-30a-5p knockout-aggravated cardiac dysfunction and heart senescence. This study identified miR-30a-5p as a crucial modulator of heart senescence and revealed that the miR-30a-5p–TP53INP1–p53 axis is essential for heart and cardiomyocyte aging. As we get older, our hearts change, leading to heart diseases, a major cause of death worldwide. A recent study looked at the role of miR-30a-5p, a tiny RNA molecule, in heart aging. Researchers found that lower levels of miR-30a-5p in the heart contribute to aging. They used genetic engineering to change miR-30a-5p levels in mice and saw how it affected heart health and aging. Lower levels of miR-30a-5p led to worse heart function, shorter telomeres, and more signs of aging. But, increasing miR-30a-5p levels protected against these effects. The study identified a protein, TP53INP1, that links miR-30a-5p to heart cell aging. The findings show that miR-30a-5p is crucial in heart aging by controlling heart cell health and lifespan. By targeting miR-30a-5p, new treatments could be made to slow heart aging and prevent related diseases. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
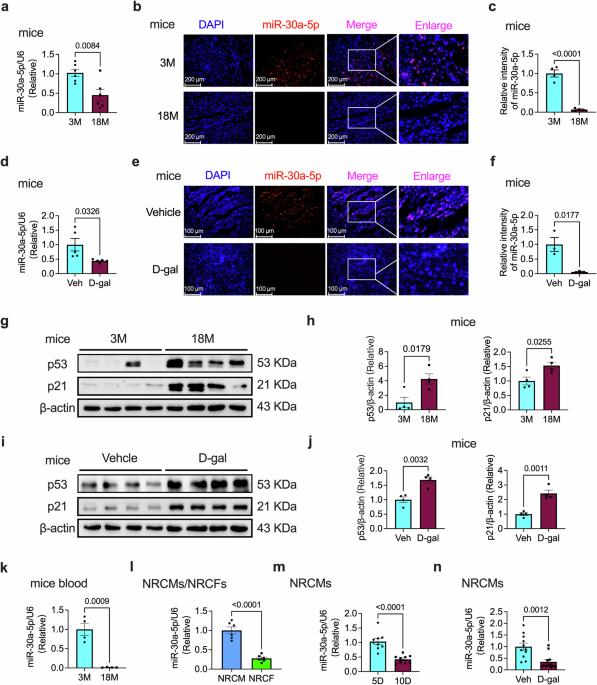
TP53INP1 的 SUMOylation 参与了 miR-30a-5p 调控的心脏衰老。
心脏衰老对心脏功能至关重要。本研究旨在描述 miR-30a-5p 在心脏衰老中的作用和作用机制。miR-30a-5p 在衰老小鼠心脏和新生大鼠心肌细胞(NRCMs)中下调。在体内,我们结合超声心动图和不同的分子生物学方法,研究了 miR-30a-5p 基因敲除或过表达在天然或 D-半乳糖诱导的小鼠心脏衰老中的作用。与野生型同系小鼠相比,miR-30a-5p 基因敲除小鼠表现出自然或 D-半乳糖诱导的心脏衰老加剧,心脏功能显著下降,γH2AX 阳性细胞数量增加,端粒长度减少,p21 和 p53 表达上调。利用腺相关病毒 9 在 D-半乳糖诱导的衰老野生型小鼠中敲除心脏特异性 miR-30a-5p,结果与敲除小鼠中观察到的效果相似。值得注意的是,在野生型小鼠心脏中过表达 miR-30a-5p,可通过改善心脏功能、增加端粒长度、减少 γH2AX 阳性细胞的数量以及下调 p53 和 p21 的表达,缓解 D-半乳糖诱导的心脏衰老。这在经 D-半乳糖处理或自然老化的 NRCMs 中得到了证实。从机理上讲,TP53INP1 通过介导 TP53INP1 的 SUMOylation 及其从细胞质转位到细胞核与 p53 相互作用,被确定为 miR-30a-5p 的靶标。此外,这项研究还证明,心脏特异性 TP53INP1 缺乏可改善 miR-30a-5p 基因敲除导致的心脏功能障碍和心脏衰老。这项研究确定了 miR-30a-5p 是心脏衰老的关键调节因子,并揭示了 miR-30a-5p-TP53INP1-p53 轴对心脏和心肌细胞衰老至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


