Cefiderocol has immunoregulative effects in LPS-induced vitro experimental model via inhibiting inflammation and ferroptosis
IF 4.9
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2024-11-05
DOI:10.1016/j.ijantimicag.2024.107374
引用次数: 0
Abstract
Background
Cefiderocol is a new catecholamine-containing siderophore cephalosporin. It, however, remains unclear how cefiderocol modulates the immune response of the host.
Objectives
This study elucidated whether cefiderocol exerts immunoprotective effects in an in vitro experimental model induced with lipopolysaccharide (LPS).
Methods
Mouse macrophage RAW 264.7 cells were exposed to LPS (100 ng/mL) or LPS + cefiderocol (40 mg/L) to assess the immunomodulatory effect of cefiderocol in vitro. ELISA was performed on cell culture supernatants to estimate cytokine levels. Ferroptosis level was also quantified by detecting intracellular reactive oxygen species and iron levels through flow cytometry analysis. Malondialdehyde and glutathione (GSH) levels were estimated by ELISA. We conducted western blotting assay for evaluating key ferroptosis pathway proteins.
Results
Cefiderocol alleviated LPS-induced inflammation by reducing IL-6, TNF-α, and IL-1β production levels and enhancing the IL-10 production level. Further analysis to determine the underlying mechanism revealed that cefiderocol inhibited ferroptosis; this was confirmed by reduced reactive oxygen species, malondialdehyde, and Fe2+ ion levels; increased GSH levels; upregulated expression of solute carrier family 7 member 11, GSH peroxidase 4, nuclear factor erythroid 2-related factor 2, and ferroptosis suppressor protein 1; and downregulated expression of acyl-CoA synthetase long-chain family member 4.
Conclusions
Cefiderocol may play a key role in reducing inflammation by decreasing inflammatory cytokine release and suppressing ferroptosis.
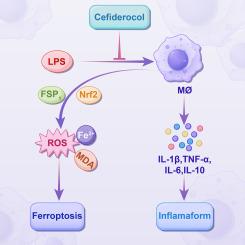
在 LPS 诱导的体外实验模型中,头孢羟氨苄通过抑制炎症和铁蛋白沉积产生免疫调节作用。
背景头孢羟氨苄是一种新的含儿茶酚胺的嗜苷头孢菌素。然而,目前仍不清楚头孢羟氨苄如何调节宿主的免疫反应:本研究阐明了头孢羟氨苄是否能在脂多糖(LPS)诱导的体外实验模型中发挥免疫保护作用:小鼠巨噬细胞 RAW 264.7 细胞暴露于 LPS(100 ng/mL)或 LPS + cefiderocol(40 mg/L),以评估 cefiderocol 在体外的免疫调节作用。对细胞培养上清液进行 ELISA 检测,以估算细胞因子水平。此外,还通过流式细胞仪分析检测细胞内活性氧(ROS)和铁水平,量化铁变态反应水平。丙二醛(MDA)和谷胱甘肽(GSH)水平是通过酶联免疫吸附法估算的。我们进行了 Western 印迹分析,以评估关键的铁变态反应通路蛋白:结果:头孢多巴通过降低IL-6、TNF-α和IL-1β的产生水平以及提高IL-10的产生水平,缓解了LPS诱导的炎症反应。为确定其潜在机制而进行的进一步分析表明,头孢可可可抑制铁变态反应;ROS、MDA 和 Fe2+ 离子水平的降低;GSH 水平的升高;溶质运载家族 7 成员 11、谷胱甘肽过氧化物酶 4、红细胞核因子 2 相关因子 2 和铁变态反应抑制蛋白 1 表达的上调;以及酰基-CoA 合成酶长链家族成员 4 表达的下调证实了这一点:结论:头孢羟氨苄可通过减少炎性细胞因子的释放和抑制铁绒毛膜促性腺激素的分泌,在减轻炎症方面发挥关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


