Purification and catalysis of choline dehydrogenase from Escherichia coli
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
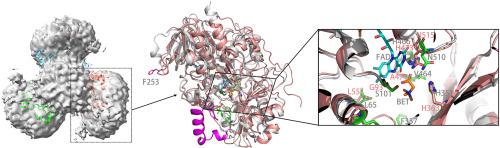
Choline dehydrogenase (CHDH) is a membrane-bound enzyme belonging to the glucose-methanol-choline (GMC) oxidoreductase superfamily, which is characterized by a crucial FAD-binding domain essential for catalytic function. CHDH catalyzes the oxidation of choline to betaine aldehyde, which is further oxidized to betaine, a vital osmoprotectant and methyl donor for cellular physiology and metabolism. However, the detailed catalytic mechanism of CHDH still remains poorly understood. In our investigation, we gained purity E. coli CHDH samples in DDM (n-dodecyl-β-D-maltoside) and SMA (styrene maleic acid) copolymer respectively and examined their structural composition and catalytic activity separately. Our findings demonstrated the effectiveness of SMA, commonly employed for extracting transmembrane proteins and can preserve the natural bio-membrane environment surrounding the enzyme, in extracting peripheral membrane proteins like CHDH here, which lacks transmembrane helices. CHDH exhibited a trimeric conformation in SMA, whereas it existed as monomers in DDM, as determined by our negative staining analysis. Our experiments also revealed that highly pure E. coli CHDH could only oxidize choline to betaine aldehyde but failed to further oxidize betaine aldehyde to betaine as determined by the biochemical and enzymatic reaction kinetic assays. In addition, the enzyme in SMA displayed greater catalytic activity compared to that in DDM. Furthermore, we confirmed the crucial role of His473, which is hypothesized to be a critical site for substrate binding from our structural comparative analysis between CHDH and its highly homologous choline oxidase, in the catalytic activity of the enzyme through gene mutation. Our work also sheds light on CHDH's contribution to cellular osmotic tolerance through gene knockout. This research enhances our better understanding of CHDH within cellular biochemistry and metabolic pathways.
大肠杆菌胆碱脱氢酶的纯化和催化。
胆碱脱氢酶(CHDH)是一种膜结合酶,属于葡萄糖-甲醇-胆碱(GMC)氧化还原酶超家族,其特点是具有对催化功能至关重要的 FAD 结合域。CHDH 催化胆碱氧化成甜菜碱醛,再进一步氧化成甜菜碱,甜菜碱是一种重要的渗透保护剂,也是细胞生理和代谢的甲基供体。然而,人们对 CHDH 的详细催化机理仍然知之甚少。在我们的研究中,我们分别在 DDM(正十二烷基-β-D-麦芽糖苷)和 SMA(苯乙烯马来酸)共聚物中获得了纯度较高的大肠杆菌 CHDH 样品,并分别考察了它们的结构组成和催化活性。我们的研究结果表明,SMA 常用于提取跨膜蛋白,并能保留酶周围的天然生物膜环境,但在提取像 CHDH 这样缺乏跨膜螺旋的外周膜蛋白时,SMA 也很有效。根据我们的阴性染色分析,CHDH 在 SMA 中呈三聚体构象,而在 DDM 中则以单体形式存在。我们的实验还发现,高纯度的大肠杆菌 CHDH 只能将胆碱氧化成甜菜碱醛,而不能进一步将甜菜碱醛氧化成甜菜碱,这是由生化和酶促反应动力学实验确定的。此外,与 DDM 中的酶相比,SMA 中的酶显示出更高的催化活性。此外,我们还通过基因突变证实了 His473 在该酶催化活性中的关键作用,根据我们对 CHDH 及其高度同源的胆碱氧化酶的结构比较分析,His473 被假定为底物结合的关键位点。我们的工作还通过基因敲除揭示了 CHDH 对细胞渗透耐受性的贡献。这项研究加深了我们对 CHDH 在细胞生物化学和代谢途径中的作用的了解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


