In-depth recognition of mixed surfactants maintaining the enzymatic activity of cellulases through stabilization of their spatial structures
IF 9.7
1区 环境科学与生态学
Q1 AGRICULTURAL ENGINEERING
引用次数: 0
Abstract
Mixed surfactants improve the enzymatic hydrolysis of lignocellulosic substrates by enhancing cellulase stability against heat, pH, shear, and air–liquid interface stress. Under conditions of multiple factorial stresses (50 °C, pH 4.8, 180 rpm, and 15.5 cm2 air–liquid interface), cellulase with ternary surfactants (Tween 60/Triton X-114/CTAB, the molar ratio 14:5.5:1) retained 84 % of its activity after 48 h of incubation, representing 1.15 and 1.29 folds that of the cellulase activity with the single Tween 60 and with no surfactants, respectively. This is attributed to the fact that ternary surfactants possess better rheology modulation and air–liquid interface competitiveness. In addition, the computational approach demonstrated that the ternary surfactants were capable of forming stronger hydrophobic and hydrogen-bond interactions with cellulase enzymes, thus maintaining its secondary structure and preventing the detrimental α-helix to β-sheet transformation known to compromise cellulase activity. This synergy offers valuable insights into surfactant-cellulase interactions and supports efficient enzymatic hydrolysis in biorefineries.
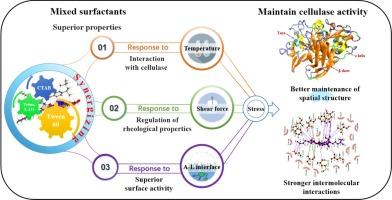
深入认识混合表面活性剂通过稳定空间结构来保持纤维素酶的酶活性。
混合表面活性剂通过提高纤维素酶对热、pH 值、剪切力和气液界面应力的稳定性来改善木质纤维素底物的酶水解。在多重因子应力(50 °C、pH 4.8、180 rpm 和 15.5 cm2 气液界面)条件下,添加三元表面活性剂(Tween 60/Triton X-114/CTAB,摩尔比为 14:5.5:1)的纤维素酶在培养 48 小时后保持了 84% 的活性,分别是添加单一 Tween 60 和不添加表面活性剂的纤维素酶活性的 1.15 倍和 1.29 倍。这是因为三元表面活性剂具有更好的流变调节性和气液界面竞争性。此外,计算方法还表明,三元表面活性剂能够与纤维素酶形成更强的疏水和氢键相互作用,从而保持其二级结构,防止已知会损害纤维素酶活性的有害的 α 螺旋向 β 片状转变。这种协同作用为了解表面活性剂与纤维素酶之间的相互作用提供了宝贵的信息,并有助于生物炼油厂进行高效的酶水解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioresource Technology
工程技术-能源与燃料
CiteScore
20.80
自引率
19.30%
发文量
2013
审稿时长
12 days
期刊介绍:
Bioresource Technology publishes original articles, review articles, case studies, and short communications covering the fundamentals, applications, and management of bioresource technology. The journal seeks to advance and disseminate knowledge across various areas related to biomass, biological waste treatment, bioenergy, biotransformations, bioresource systems analysis, and associated conversion or production technologies.
Topics include:
• Biofuels: liquid and gaseous biofuels production, modeling and economics
• Bioprocesses and bioproducts: biocatalysis and fermentations
• Biomass and feedstocks utilization: bioconversion of agro-industrial residues
• Environmental protection: biological waste treatment
• Thermochemical conversion of biomass: combustion, pyrolysis, gasification, catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


