Rescue of mitochondrial dysfunction through alteration of extracellular matrix composition in barth syndrome cardiac fibroblasts
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Fibroblast-ECM (dys)regulation is associated with a plethora of diseases. The ECM acts as a reservoir of inflammatory factors and cytokines that mediate molecular mechanisms within cardiac cell populations. The role of ECM-mitochondria crosstalk in the development and progression of cardiac disorders remains uncertain. We evaluated the influence of ECM produced by stromal cells from patients with the mitochondrial cardiomyopathy (Barth syndrome, BTHS) and unaffected healthy controls on cardiac fibroblast (CF) metabolic function. To do this, cell-derived matrices CDMs were generated from BTHS and healthy human pluripotent stem cell-derived CFs (hPSC-CF) and used as cell culture substrates. BTHS CDMs negatively impacted the mitochondrial function of healthy hPSC-CFs while healthy CDMs improved mitochondrial function in BTHS hPSC-CFs. Mass spectrometry comparisons identified 5 matrisome proteins differentially expressed in BTHS compared to healthy CDM. Our results highlight a key role for the ECM in disease through its impact on mitochondrial function.
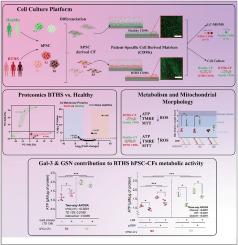
通过改变巴氏综合征心脏成纤维细胞的细胞外基质组成来挽救线粒体功能障碍。
成纤维细胞-ECM(失调)与多种疾病相关。ECM 是炎症因子和细胞因子的储存库,可介导心脏细胞群内的分子机制。ECM 与线粒体之间的相互作用在心脏疾病的发生和发展中的作用仍不确定。我们评估了线粒体心肌病(巴特综合征,BTHS)患者和未受影响的健康对照组的基质细胞产生的 ECM 对心脏成纤维细胞(CF)代谢功能的影响。为此,从 BTHS 和健康人多能干细胞衍生的成纤维细胞(hPSC-CF)中生成了细胞衍生基质 CDMs,并将其用作细胞培养基质。BTHS CDMs 对健康 hPSC-CFs 的线粒体功能产生了负面影响,而健康 CDMs 则改善了 BTHS hPSC-CFs 的线粒体功能。质谱比较发现,与健康 CDM 相比,5 种 matrisome 蛋白在 BTHS 中表达不同。我们的研究结果凸显了 ECM 通过影响线粒体功能在疾病中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


