Enhancing the Stability of Cu-Based Electrocatalyst via Fe Alloy in Electrocatalytic Formaldehyde Oxidation with Long Durability
IF 5.1
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
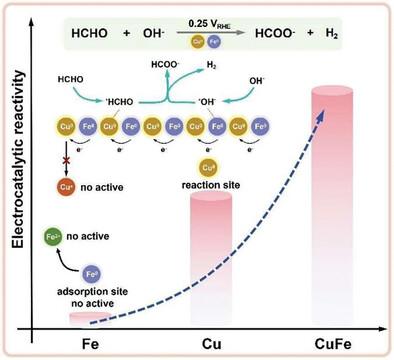
Electrocatalytic formaldehyde oxidation with metal Cu electrocatalyst has attracted significant interest since it can produce H2 at the anode and make it possible to construct a bipolar hydrogen production cell with low voltage. However, the activity of the Cu electrocatalyst will be greatly weakened after oxidizing it to Cu+ or Cu2+. Here, a CuFe bimetallic catalyst is developed to efficiently catalyze the electro-oxidation process of HCHO to produce H2 at a potential of 0.10 VRHE with a current density of 100 mA cm−2. It is confirmed that introducing Fe in a CuFe catalyst can regulate the electron configuration to prevent Cu0 oxidation and improve the stability of the catalysts. The introduction of Fe can reduce the energy barrier of the reaction process, and make the C─H bond more easily split on CuFe. A bipolar hydrogen production device is constructed by combining the anodic oxidation of HCHO with the cathodic hydrogen evolution. The current density of 500 mA cm−2 is achieved at a cell voltage of 0.6 V. The faradaic efficiency is ≈100% and the device is stable for ≈50 h. The research provides a promising path toward the secure, effective, and expandable generation of high-purity H2 at both anodic and cathodic electrodes.
通过铁合金提高铜基电催化剂在电催化甲醛氧化中的稳定性并延长其使用寿命
使用金属 Cu 电催化剂进行电催化甲醛氧化引起了人们的极大兴趣,因为它可以在阳极产生 H2,并使低电压双极制氢电池的构建成为可能。然而,Cu 电催化剂在氧化成 Cu+ 或 Cu2+ 后,其活性会大大减弱。本文开发了一种 CuFe 双金属催化剂,可在 0.10 VRHE 的电位和 100 mA cm-2 的电流密度下高效催化 HCHO 的电氧化过程以产生 H2。研究证实,在 CuFe 催化剂中引入 Fe 可以调节电子构型,防止 Cu0 氧化,提高催化剂的稳定性。铁的引入可以降低反应过程的能障,使 C─H 键更容易在 CuFe 上分裂。通过将 HCHO 的阳极氧化与阴极氢气进化相结合,构建了一种双极制氢装置。该研究为在阳极和阴极安全、有效和可扩展地生成高纯度 H2 提供了一条可行之路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


