Synthesis of 4-Hydroxyindolin-2-ones via Phosphoric Acid-Mediated Annulation of β-Nitrostyrenes with 1,3-Cyclohexanedione
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The efficient synthesis of 4-hydroxy-3-arylindolin-2-ones via phosphoric acid-mediated annulation of various β-nitrostyrenes and 1,3-cyclohexanedione is described. This annulation reaction gives a practical method for affording a diverse set of oxindoles, having simple experimentation, readily available starting materials, and very good yields. Additionally, substituted 1,3-cyclohexanediones under the same conditions afforded tetrahydrobenzofuran oxime compounds.
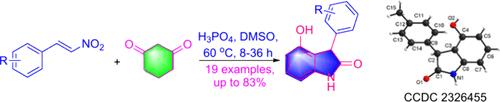
通过磷酸介导的 β-硝基苯炔与 1,3-环己二酮的环化反应合成 4-羟基吲哚啉-2-酮
本文介绍了通过磷酸介导的各种 β-硝基苯炔和 1,3-环己二酮环化反应高效合成 4-羟基-3-芳基吲哚啉-2-酮的方法。这种环化反应提供了一种获得多种羰基吲哚的实用方法,实验简单,起始原料容易获得,而且收率非常高。此外,在相同的条件下,取代的 1,3-环己二酮还能得到四氢苯并呋喃肟化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


